Chapter 2.4
Class 4 - Flammable solids;
substances liable to spontaneous combustion;
substances which, in contact with water, emit flammable gases
- 2.4.0
-
Introductory note
Since organometallic substances can be classified in classes 4.2 or 4.3 with additional subsidiary hazards, depending on their properties, a specific classification flowchart for these substances is given in 2.4.5.
- 2.4.1
- Definition and general provisions
- 2.4.1.1
-
In this Code, class 4 deals with substances, other than those classified as explosives, which, under conditions of transport, are readily combustible or may cause or contribute to a fire. Class 4 is subdivided as follows:
Class 4.1 - Flammable solids
Solids which, under conditions encountered in transport, are readily combustible or may cause or contribute to fire through friction; self-reactive substances (solids and liquids) and polymerizing substances which are liable to undergo a strongly exothermic reaction; solid desensitized explosives which may explode if not diluted sufficiently;Class 4.2 - Substances liable to spontaneous combustion
Substances (solids and liquids) which are liable to spontaneous heating under normal conditions encountered in transport, or to heating up in contact with air, and being then liable to catch fire;Class 4.3 - Substances which, in contact with water, emit flammable gases
Substances (solids and liquids) which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities. - 2.4.1.2
-
As referenced in this chapter, test methods and criteria, with advice on application of the tests, are given in the Manual of Tests and Criteria for the classification of following types of substances of class 4:
flammable solids (class 4.1);
self-reactive substances (class 4.1);
polymerizing substances (class 4.1);
pyrophoric solids (class 4.2);
pyrophoric liquids (class 4.2);
self-heating substances (class 4.2); and
substances which, in contact with water, emit flammable gases (class 4.3).
Test methods and criteria for self-reactive substances and polymerizing substances are given in part II of the Manual of Tests and Criteria, and test methods and criteria for the other types of substances of class 4 are given in the Manual of Tests and Criteria, part III, section 33.
- 2.4.2
- Class 4.1 - Flammable solids, self-reactive substances, solid desensitized explosives and polymerizing substances
- 2.4.2.1
-
General
Class 4.1 includes the following types of substances:
flammable solids (see 2.4.2.2);
self-reactive substances (see 2.4.2.3);
solid desensitized explosives (see 2.4.2.4); and
polymerizing substances (see 2.4.2.5).
Some substances (such as celluloid) may evolve toxic and flammable gases when heated or if involved in a fire.
- 2.4.2.2
- Class 4.1 Flammable solids
- 2.4.2.2.1
- Definitions and properties
- 2.4.2.2.1.1
- For the purpose of this Code, flammable solids means readily combustible solids and solids which may cause fire through friction.
- 2.4.2.2.1.2
- Readily combustible solids means fibres, powdered, granular, or pasty substances which are dangerous if they can be easily ignited by brief contact with an ignition source such as a burning match, and if the flame spreads rapidly. The danger may come not only from the fire but also from toxic combustion products. Metal powders are especially dangerous because of the difficulty of extinguishing a fire, since normal extinguishing agents such as carbon dioxide or water can increase the hazard.
- 2.4.2.2.2
- Classification of flammable solids
- 2.4.2.2.2.1
- Powdered, granular or pasty substances shall be classified as readily combustible solids of class 4.1 when the time of burning of one or more of the test runs, performed in accordance with the test method described in the Manual of Tests and Criteria, part III, sub-section 33.2, is less than 45 s or the rate of burning is more than 2.2 mm/s. Powders of metals or metal alloys shall be classified in class 4.1 when they can be ignited and the reaction spreads over the whole length of the sample in 10 min or less.
- 2.4.2.2.2.2
- Solids which may cause fire through friction shall be classified in class 4.1 by analogy with existing entries (such as matches) until definitive criteria are established.
- 2.4.2.2.3
- Assignment of packing groups
- 2.4.2.2.3.1
- Packing groups are assigned on the basis of the test methods referred to in 2.4.2.2.2.1. For readily combustible solids (other than metal powders), packing group II shall be assigned if the burning time is less than 45 s and the flame passes the wetted zone. Packing group II shall be assigned to powders of metal or metal alloys if the zone of reaction spreads over the whole length of the sample in five minutes or less.
- 2.4.2.2.3.2
- Packing groups are assigned on the basis of the test methods referred to in 2.4.2.2.2.1. For readily combustible solids (other than metal powders), packing group III shall be assigned if the burning time is less than 45 s and the wetted zone stops the flame propagation for at least four minutes. Packing group III shall be assigned to metal powders if the reaction spreads over the whole length of the sample in more than five minutes but not more than 10 min.
- 2.4.2.2.3.3
- For solids which may cause fire through friction, the packing group shall be assigned by analogy with existing entries or in accordance with any appropriate special provision.
- 2.4.2.2.4
- Pyrophoric metal powders, if wetted with sufficient water to suppress their pyrophoric properties, may be classified as class 4.1.
- 2.4.2.3
- Class 4.1 Self-reactive substances
- 2.4.2.3.1
- Definitions and properties
- 2.4.2.3.1.1
-
For the purposes of this Code:
Self-reactive substances are thermally unstable substances liable to undergo a strongly exothermic decomposition even without participation of oxygen (air). Substances are not considered to be self-reactive substances of class 4.1, if:
they are explosives according to the criteria of class 1;
they are oxidizing substances according to the classification procedure for class 5.1 (see 2.5.2 except that mixtures of oxidizing substances which contain 5.0% or more of combustible organic substances shall be subjected to the classification procedure defined in note 3;
they are organic peroxides according to the criteria of class 5.2;
their heat of decomposition is less than 300 J/g; or
their self-accelerating decomposition temperature (SADT) (see 2.4.2.3.4) is greater than 75°C for a 50 kg package.
Note 1: The heat of decomposition may be determined using any internationally recognized method such as differential scanning calorimetry and adiabatic calorimetry.
Note 2: Any substance which shows the properties of a self-reactive substance shall be classified as such, even if this substance gives a positive test result according to 2.4.3.2 for inclusion in class 4.2.
Note 3: Mixtures of oxidizing substances meeting the criteria of class 5.1 which contain 5.0% or more of combustible organic substances, which do not meet the criteria mentioned in .1, .3, .4 or .5 above, shall be subjected to the self-reactive substance classification procedure.
A mixture showing the properties of a self-reactive substance, types B to F, shall be classified as a self-reactive substance of class 4.1.
A mixture showing the properties of a self-reactive substance, type G, according to the principle of 2.4.2.3.3.2.7 shall be considered for classification as a substance of class 5.1 (see 2.5.2).
- 2.4.2.3.1.2
-
The decomposition of self-reactive substances can be initiated by heat, contact with catalytic impurities (such as acids, heavy-metal compounds, bases), friction or impact. The rate of decomposition increases with temperature and varies with the substance. Decomposition, particularly if no ignition occurs, may result in the evolution of toxic gases or vapours. For certain self-reactive substances, the temperature shall be controlled. Some self-reactive substances may decompose explosively, particularly if confined. This characteristic may be modified by the addition of diluents or by the use of appropriate packagings. Some self-reactive substances burn vigorously. Self-reactive substances are, for example, some compounds of the types listed below:
aliphatic azo compounds (-C-N=N-C-);
organic azides (-C-N3);
diazonium salts (-CN2+ Z-);
N-nitroso compounds (-N-N=O); and
aromatic sulphonylhydrazides (-SO2-NH-NH2).
This list is not exhaustive and substances with other reactive groups and some mixtures of substances may have similar properties.
- 2.4.2.3.2
- Classification of self-reactive substances
- 2.4.2.3.2.1
- Self-reactive substances are classified into seven types according to the degree of danger they present. The types of self-reactive substance range from type A, which may not be accepted for transport in the packaging in which it is tested, to type G, which is not subject to the provisions for self-reactive substances of class 4.1. The classification of types B to F is directly related to the maximum quantity allowed in one packaging.
- 2.4.2.3.2.2
-
Self-reactive substances permitted for transport in packagings are listed in 2.4.2.3.2.3, those permitted for transport in IBCs are listed in packing instruction IBC520 and those permitted for transport in portable tanks are listed in portable tank instruction T23. For each permitted substance listed, the appropriate generic entry of the Dangerous Goods List (UN 3221 to UN 3240) is assigned, and appropriate subsidiary hazards and remarks providing relevant transport information are given. The generic entries specify:
self-reactive substance type (B to F);
physical state (liquid or solid); and
temperature control, when required (2.4.2.3.4).
- 2.4.2.3.2.3
-
List of currently assigned self-reactive substances in packagings
In the column "Packing Method" codes "OP1" to "OP8" refer to packing methods in packing instruction P520. Self-reactive substances to be transported shall fulfil the classification and the control and emergency temperatures (derived from the SADT) as listed. For substances permitted in IBCs, see packing instruction IBC520, and for those permitted in tanks, see portable tank instruction T23. The formulations not listed in this provision but listed in packing instruction IBC520 of 4.1.4.2 and in portable tank instruction T23 of 4.2.5.2.6 may also be transported packed in accordance with packing method OP8 of packing instruction P520 of 4.1.4.1, with the same control and emergency temperatures, if applicable.
Note: The classification given in this table is based on the technically pure substance (except where a concentration of less than 100% is specified). For other concentrations, the substances may be classified differently following the procedures in 2.4.2.3.3 and 2.4.2.3.4.
UN generic entry SELF-REACTIVE SUBSTANCE Concentration (%) Packing method Control temperature (°C) Emergency temperature (°C) Remarks 3222 2-DIAZO-1-NAPHTHOL-4-SULPHONYL CHLORIDE 100 OP5 (2) 2-DIAZO-1-NAPHTHOL-5-SULPHONYL CHLORIDE 100 OP5 (2) 3223 SELF-REACTIVE LIQUID, SAMPLE OP2 (8) 3224 AZODICARBONAMIDE FORMULATION TYPE C < 100 OP6 (3) 2,2'-AZODI(ISOBUTYRONITRILE) as a water-based paste ≤ 50 OP6 N,N'-DINITROSO-N,N'- DIMETHYLTEREPHTHALAMIDE, as a paste 72 OP6 N,N'-DINITROSOPENTAMETHYLENETETRAMINE 82 OP6 (7) SELF-REACTIVE SOLID, SAMPLE OP2 (8) 3226 AZODICARBONAMIDE FORMULATION TYPE D < 100 OP7 (5) 1,1'-AZODI(HEXAHYDROBENZONITRILE) 100 OP7 BENZENE-1,3-DISULPHONYL HYDRAZIDE as a paste 52 OP7 BENZENESULPHONYL HYDRAZIDE 100 OP7 4-(BENZYL(ETHYL)AMINO)-3- ETHOXYBENZENEDIAZONIUM ZINC CHLORIDE 100 OP7 3-CHLORO-4-DIETHYLAMINOBENZENEDIAZONIUM ZINC CHLORIDE 100 OP7 2-DIAZO-1-NAPHTHOLSULPHONIC ACID ESTER MIXTURE TYPE D < 100 OP7 (9) 2,5-DIETHOXY-4-(4-MORPHOLINYL) BENZENEDIAZONIUM SULPHATE 100 OP7 DIPHENYLOXIDE-4,4'-DISULPHONYL HYDRAZIDE 100 OP7 4-DIPROPYLAMINOBENZENEDIAZONIUM ZINC CHLORIDE 100 OP7 4-METHYLBENZENESULPHONYLHYDRAZIDE 100 OP7 SODIUM 2-DIAZO-1-NAPHTHOL-4-SULPHONATE 100 OP7 SODIUM 2-DIAZO-1-NAPHTHOL-5-SULPHONATE 100 OP7 3227 PHOSPHOROTHIOIC ACID, O-[(CYANOPHENYL 82-91 | O P8 (10) METHYLENE) AZANYL] O,O-DIETHYL ESTER (Z isomer) 3228 ACETONE-PYROGALLOL COPOLYMER 2-DIAZO-1- NAPHTHOL-5-SULPHONATE 100 OP8 4-(DIMETHYLAMINO)BENZENEDIAZONIUM TRICHLOROZINCATE(-1) 100 OP8 2,5-DIBUTOXY-4-(4-MORPHOLINYL)- BENZENEDIAZONIUM TETRACHLOROZINCATE(2:1) 100 OP8 3230 (7-METHOXY-5-METHYL-BENZOTHIOPHEN-2-YL) BORONIC ACID 88- 100 OP7 (11) 3232 AZODICARBONAMIDE FORMULATION TYPE B, TEMPERATURE CONTROLLED < 100 OP5 (1) (2) 3233 SELF-REACTIVE LIQUID, SAMPLE, TEMPERATURE CONTROLLED OP2 (8) 3234 AZODICARBONAMIDE FORMULATION TYPE C, TEMPERATURE CONTROLLED < 100 OP6 (4) 2,2'-AZODI(ISOBUTYRONITRILE) 100 OP6 +40 +45 3-METHYL-4-(PYRROLIDIN-1-YL) BENZENEDIAZONIUM TETRAFLUOROBORATE 95 OP6 +45 +50 SELF-REACTIVE SOLID, SAMPLE, TEMPERATURE CONTROLLED OP2 (8) TETRAMINEPALLADIUM(II) NITRATE 100 OP6 +30 +35 3235 2,2'-AZODI(ETHYL-2-METHYLPROPIONATE) 100 OP7 +20 +25 3236 AZODICARBONAMIDE FORMULATION TYPE D, TEMPERATURE CONTROLLED < 100 OP7 (6) 2,2'-AZODI(2,4-DIMETHYL-4- METHOXYVALERONITRILE) 100 OP7 -5 +5 2,2'-AZODI(2,4-DIMETHYLVALERONITRILE) 100 OP7 +10 +15 2,2'-AZODI(2-METHYLBUTYRONITRILE) 100 OP7 +35 +40 4-(BENZYL(METHYL)AMINO)-3- ETHOXYBENZENEDIAZONIUM ZINC CHLORIDE 100 OP7 +40 +45 2,5-DIETHOXY-4-MORPHOLINOBENZENEDIAZONIUM ZINC CHLORIDE 67-100 OP7 +35 +40 2,5-DIETHOXY-4-MORPHOLINOBENZENEDIAZONIUM ZINC CHLORIDE 66 OP7 +40 +45 2,5-DIETHOXY-4-MORPHOLINOBENZENEDIAZONIUM TETRAFLUOROBORATE 100 OP7 +30 +35 2,5-DIETHOXY-4-(PHENYLSULPHONYL) BENZENEDIAZONIUM ZINC CHLORIDE 67 OP7 +40 +45 2,5-DIMETHOXY-4-(4-METHYLPHENYLSULPHONYL) BENZENEDIAZONIUM ZINC CHLORIDE 79 OP7 +40 +45 4-DIMETHYLAMINO-6-(2-DIMETHYLAMINOETHOXY) TOLUENE-2-DIAZONIUM ZINC CHLORIDE 100 OP7 +40 +45 2-(N,N-ETHOXYCARBONYLPHENYLAMINO)-3- METHOXY-4-(N-METHYL-N-CYCLOHEXYLAMINO) BENZENEDIAZONIUM ZINC CHLORIDE 63-92 OP7 +40 +45 2-(N,N-ETHOXYCARBONYLPHENYLAMINO)-3- METHOXY-4-(N-METHYL-N-CYCLOHEXYLAMINO) BENZENEDIAZONIUM ZINC CHLORIDE 62 OP7 +35 +40 N-FORMYL-2-(NITROMETHYLENE)-1,3- PERHYDROTHIAZINE 100 OP7 +45 +50 2-(2-HYDROXYETHOXY)-1-(PYRROLIDIN-1-YL) BENZENE-4-DIAZONIUM ZINC CHLORIDE 100 OP7 +45 +50 3-(2-HYDROXYETHOXY)-4-(PYRROLIDIN-1-YL) BENZENEDIAZONIUM ZINC CHLORIDE 100 OP7 +40 +45 2-(N,N-METHYLAMINOETHYLCARBONYL)- 4-(3,4-DIMETHYLPHENYLSULPHONYL)- BENZENEDIAZONIUM HYDROGEN SULPHATE 96 OP7 +45 +50 4-NITROSOPHENOL 100 OP7 +35 +40 3237 DIETHYLENEGLYCOL BIS(ALLYLCARBONATE) + DI-ISOPROPYL PEROXYDICARBONATE ≥ 88 + ≤ 12 OP8 -10 0 Remarks
- Azodicarbonamide formulations which fulfil the criteria of 2.4.2.3.3.2.2. The control and emergency temperatures shall be determined by the procedure given in 7.3.7.2
- "EXPLOSIVE" subsidiary hazard label required (Model No 1, see 5.2.2.2.2).
- Azodicarbonamide formulations which fulfil the criteria of 2.4.2.3.3.2.3.
- Azodicarbonamide formulations which fulfil the criteria of 2.4.2.3.3.2.3. The control and emergency temperatures shall be determined by the procedure given in 7.3.7.2
- Azodicarbonamide formulations which fulfil the criteria of 2.4.2.3.3.2.4.
- Azodicarbonamide formulations which fulfil the criteria of 2.4.2.3.3.2.4. The control and emergency temperatures shall be determined by the procedure given in 7.3.7.2.
- With a compatible diluent having a boiling point of not less than 150°C.
- See 2.4.2.3.2.4.2.
- This entry applies to mixtures of esters of 2-diazo-1-naphthol-4-sulphonic acid and 2-diazo-1-naphthol-5-sulphonic acid meeting the criteria of 2.4.2.3.3.2.4.
- This entry applies to the technical mixture in n-butanol within the specified concentration limits of the (Z) isomer.
- The technical compound with the specified concentration limits may contain up to 12% water and up to 1% organic impurities.
- 2.4.2.3.2.4
-
Classification of self-reactive substances not listed in 2.4.2.3.2.3, packing instruction IBC520 or portable tank instruction T23 and assignment to a generic entry shall be made by the competent authority of the country of origin on the basis of a test report. Principles applying to the classification of such substances are provided in 2.4.2.3.3. The applicable classification procedures, test methods and criteria, and an example of a suitable test report, are given in the Manual of Tests and Criteria, part II. The statement of approval shall contain the classification and the relevant transport conditions.
Activators, such as zinc compounds, may be added to some self-reactive substances to change their reactivity. Depending on both the type and the concentration of the activator, this may result in a decrease in thermal stability and a change in explosive properties. If either of these properties is altered, the new formulation shall be assessed in accordance with this classification procedure.
Samples of self-reactive substances or formulations of self-reactive substances not listed in 2.4.2.3.2.3, for which a complete set of test results is not available and which are to be transported for further testing or evaluation, may be assigned to one of the appropriate entries for self-reactive substances type C provided the following conditions are met:
the available data indicate that the sample would be no more dangerous than self-reactive substances type B;
the sample is packaged in accordance with packing method OP2 (see applicable packing instruction) and the quantity per cargo transport unit is limited to 10 kg; and
the available data indicate that the control temperature, if any, is sufficiently low to prevent any dangerous decomposition and sufficiently high to prevent any dangerous phase separation.
- 2.4.2.3.3
-
Principles for classification of self-reactive substances
Note: This section refers only to those properties of self-reactive substances which are decisive for their classification. A flow chart, presenting the classification principles in the form of a graphically arranged scheme of questions concerning the decisive properties together with the possible answers, is given in Figure 2.4.1 in chapter 2.4 of the Recommendations on the Transport of Dangerous Goods. These properties shall be determined experimentally. Suitable test methods with pertinent evaluation criteria are given in the Manual of Tests and Criteria, part II.
- 2.4.2.3.3.1
- A self-reactive substance is regarded as possessing explosive properties when, in laboratory testing, the formulation is liable to detonate, to deflagrate rapidly or to show a violent effect when heated under confinement.
- 2.4.2.3.3.2
-
The following principles apply to the classification of self-reactive substances not listed in 2.4.2.3.2.3:
Any substance which can detonate or deflagrate rapidly, as packaged for transport, is prohibited from transport under the provisions for self-reactive substances of class 4.1 in that packaging (defined as SELF-REACTIVE SUBSTANCE TYPE A);
Any substance possessing explosive properties and which, as packaged for transport, neither detonates nor deflagrates rapidly, but is liable to undergo a thermal explosion in that package, shall also bear an "EXPLOSIVE" subsidiary hazard label (Model No. 1, see 5.2.2.2.2). Such a substance may be packaged in amounts of up to 25 kg unless the maximum quantity has to be limited to a lower amount to preclude detonation or rapid deflagration in the package (defined as SELF-REACTIVE SUBSTANCE TYPE B);
Any substance possessing explosive properties may be transported without an "EXPLOSIVE" subsidiary hazard label when the substance as packaged (maximum 50 kg) for transport cannot detonate or deflagrate rapidly or undergo a thermal explosion (defined as SELF-REACTIVE SUBSTANCE TYPE C);
Any substance which, in laboratory testing:
detonates partially, does not deflagrate rapidly and shows no violent effect when heated under confinement; or
does not detonate at all, deflagrates slowly and shows no violent effect when heated under confinement; or
does not detonate or deflagrate at all and shows a medium effect when heated under confinement may be accepted for transport in packages of not more than 50 kg net mass (defined as SELF-REACTIVE SUBSTANCE TYPE D);
Any substance which, in laboratory testing, neither detonates nor deflagrates at all and shows low or no effect when heated under confinement may be accepted for transport in packages of not more than 400 kg/450 L (defined as SELF-REACTIVE SUBSTANCE TYPE E);
Any substance which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows only a low or no effect when heated under confinement as well as low or no explosive power may be considered for transport in IBCs (defined as SELF-REACTIVE SUBSTANCE TYPE F); (for additional provisions see 4.1.7.2.2);
Any substance which, in laboratory testing, neither detonates in the cavitated state nor deflagrates at all and shows no effect when heated under confinement nor any explosive power shall be exempted from classification as a self-reactive substance of class 4.1 provided that the formulation is thermally stable (self-accelerating decomposition temperature 60°C to 75°C for a 50 kg package) and any diluent meets the provisions of 2.4.2.3.5 (defined as SELF-REACTIVE SUBSTANCE TYPE G). If the formulation is not thermally stable or a compatible diluent having a boiling point less than 150°C is used for desensitization, the formulation shall be defined as SELF-REACTIVE LIQUID/SOLID TYPE F.
- 2.4.2.3.4
- Temperature control provisions
- 2.4.2.3.4.1
- Self-reactive substances are subject to temperature control in transport if their self-accelerating decomposition temperature (SADT) is less than or equal to 55°C. For currently assigned self-reactive substances, the control and emergency temperatures are shown in 2.4.2.3.2.3. Test methods for determining the SADT are given in the Manual of Tests and Criteria, part II, section 28. The test selected shall be conducted in a manner which is representative, both in size and material, of the package to be transported. The temperature control provisions are given in 7.3.7.
- 2.4.2.3.5
- Desensitization of self-reactive substances
- 2.4.2.3.5.1
- In order to ensure safety during transport, self-reactive substances may be desensitized through the use of a diluent. If a diluent is used, the self-reactive substance shall be tested with the diluent present in the concentration and form used in transport.
- 2.4.2.3.5.2
- Diluents which may allow a self-reactive substance to concentrate to a dangerous extent in the event of leakage from a package shall not be used.
- 2.4.2.3.5.3
- The diluent shall be compatible with the self-reactive substance. In this regard, compatible diluents are those solids or liquids which have no detrimental influence on the thermal stability and hazard type of the self-reactive substance.
- 2.4.2.3.5.4
- Liquid diluents in liquid formulations requiring temperature control shall have a boiling point of at least 60°C and a flashpoint not less than 5°C. The boiling point of the liquid shall be at least 50°C higher than the control temperature of the self-reactive substance (see 7.3.7.2).
- 2.4.2.4
- Class 4.1 Solid desensitized explosives
- 2.4.2.4.1
- Definitions and properties
- 2.4.2.4.1.1
- Solid desensitized explosives are explosive substances which are wetted with water or alcohols or are diluted with other substances to form a homogeneous solid mixture to suppress their explosive properties. The desensitizing agent shall be distributed uniformly throughout the substance in the state in which it is to be transported. Where transport under conditions of low temperature is anticipated for substances containing or wetted with water, a suitable and compatible solvent, such as alcohol, may have to be added to lower the freezing point of the liquid. Some of these substances, when in a dry state, are classified as explosives. Where reference is made to a substance which is wetted with water, or some other liquid, it shall be permitted for transport as a class 4.1 substance only when in the wetted condition specified. Entries in the Dangerous Goods List in chapter 3.2 for solid desensitized explosives are UN 1310, UN 1320, UN 1321, UN 1322, UN 1336, UN 1337, UN 1344, UN 1347, UN 1348, UN 1349, UN 1354, UN 1355, UN 1356, UN 1357, UN 1517, UN 1571, UN 2555, UN 2556, UN 2557, UN 2852, UN 2907, UN 3317, UN 3319, UN 3344, UN 3364, UN 3365, UN 3366, UN 3367, UN 3368, UN 3369, UN 3370, UN 3376, UN 3380 and UN 3474.
- 2.4.2.4.2
-
Substances that:
have been provisionally accepted into class 1 according to test series 1 and 2 but exempted from class 1 by test series 6;
are not self-reactive substances of class 4.1;
are not substances of class 5
are also assigned to class 4.1. UN 2956, UN 3241, UN 3242 and UN 3251 are such entries.
- 2.4.2.5
- Class 4.1 Polymerizing substances and mixtures (stabilized)
- 2.4.2.5.1
-
Definitions and properties
Polymerizing substances are substances which, without stabilization, are liable to undergo a strongly exothermic reaction resulting in the formation of larger molecules or resulting in the formation of polymers under conditions normally encountered in transport. Such substances are considered to be polymerizing substances of class 4.1 when:
their self-accelerating polymerization temperature (SAPT) is 75°C or less under the conditions (with or without chemical stabilization as offered for transport) and in the packaging, IBC or portable tank in which the substance or mixture is to be transported;
they exhibit a heat of reaction of more than 300 J/g; and
they do not meet any other criteria for inclusion in classes 1 to 8.
A mixture meeting the criteria of a polymerizing substance shall be classified as a polymerizing substance of Class 4.1.
- 2.4.2.5.2
-
Polymerizing substances are subject to temperature control in transport if their self-accelerating polymerization temperature (SAPT) is:
when offered for transport in a packaging or IBC, 50°C or less in the packaging or IBC in which the substance is to be transported; or
when offered for transport in a portable tank, 45°C or less in the portable tank in which the substance is to be transported.
Note: Substances meeting the criteria of a polymerizing substance and also for inclusion in classes 1 to 8 are subject to the requirements of special provision 386 of chapter 3.3.
- 2.4.3
- Class 4.2 - Substances liable to spontaneous combustion
- 2.4.3.1
- Definitions and properties
- 2.4.3.1.1
-
Class 4.2 comprises:
Pyrophoric substances, which are substances, including mixtures and solutions (liquid or solid), which, even in small quantities, ignite within 5 minutes of coming into contact with air. These substances are the most liable to spontaneous combustion; and
Self-heating substances, which are substances, other than pyrophoric substances, which, in contact with air without energy supply, are liable to self-heating. These substances will ignite only when in large amounts (kilograms) and after long periods of time (hours or days).
- 2.4.3.1.2
- Self-heating of a substance is a process where the gradual reaction of that substance with oxygen (in air) generates heat. If the rate of heat production exceeds the rate of heat loss, then the temperature of the substance will rise which, after an induction time, may lead to self-ignition and combustion.
- 2.4.3.1.3
- Some substances may also give off toxic gases if involved in a fire.
- 2.4.3.2
- Classification of class 4.2 substances
- 2.4.3.2.1
- Solids are considered pyrophoric solids which shall be classified in class 4.2 if, in tests performed in accordance with the test method given in the Manual of Tests and Criteria, part III, sub-section 33.4.4, the sample ignites in one of the tests.
- 2.4.3.2.2
- Liquids are considered pyrophoric liquids which shall be classified in class 4.2 if, in tests performed in accordance with the test method given in the Manual of Tests and Criteria, part III, sub-section 33.4.5, the liquid ignites in the first part of the test, or if it ignites or chars the filter paper.
- 2.4.3.2.3
- Self-heating substances
- 2.4.3.2.3.1
-
A substance shall be classified as a self-heating substance of class 4.2 if, in tests performed in accordance with the test method given in the Manual of Tests and Criteria, part III, sub-section 33.4.6:
a positive result is obtained using a 25 mm cube sample at 140°C;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 100 mm cube sample at 120°C and the substance is to be transported in packages with a volume of more than 3 m³;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 100 mm cube sample at 100°C and the substance is to be transported in packages with a volume of more than 450 L;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a positive result is obtained using a 100 mm cube sample at 100°C.
Note: Self-reactive substances, giving also a positive result with this test method shall not be classified in class 4.2 but in class 4.1 (see 2.4.2.3.1.1).
- 2.4.3.2.3.2
-
A substance shall not be classified in class 4.2 if:
a negative result is obtained in a test using a 100 mm cube sample at 140°C;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C, a negative result is obtained in a test using a 100 mm cube sample at 120°C and the substance is to be transported in packages with a volume not more than 3 m3;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C, a negative result is obtained in a test using a 100 mm cube sample at 100°C and the substance is to be transported in packages with a volume not more than 450 L.
- 2.4.3.3
- Assignment of packing groups
- 2.4.3.3.1
- Packing group I shall be assigned to all pyrophoric solids and liquids.
- 2.4.3.3.2
- Packing group II shall be assigned to self-heating substances which give a positive result in a test using a 25 mm cube sample at 140°C.
- 2.4.3.3.3
-
Packing group III shall be assigned to self-heating substances if:
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C and the substance is to be transported in packages with a volume of more than 3 m3;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C, a positive result is obtained in a test using a 100 mm cube sample at 120°C and the substance is to be transported in packages with a volume of more than 450 L;
a positive result is obtained in a test using a 100 mm cube sample at 140°C and a negative result is obtained in a test using a 25 mm cube sample at 140°C and a positive result is obtained in a test using a 100 mm cube sample at 100°C.
- 2.4.4
- Class 4.3 - Substances which, in contact with water, emit flammable gases
- 2.4.4.1
- Definitions and properties
- 2.4.4.1.1
- For the purpose of this Code, the substances in this class are either liquids or solids which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities.
- 2.4.4.1.2
- Certain substances, in contact with water, may emit flammable gases that can form explosive mixtures with air. Such mixtures are easily ignited by all ordinary sources of ignition, for example naked lights, sparking handtools or unprotected lamps. The resulting blast wave and flames may endanger people and the environment. The test method referred to in 2.4.4.2 is used to determine whether the reaction of a substance with water leads to the development of a dangerous amount of gases which may be flammable. This test method shall not be applied to pyrophoric substances.
- 2.4.4.2
- Classification of class 4.3 substances
- 2.4.4.2.1
-
Substances which, in contact with water, emit flammable gases shall be classified in class 4.3 if, in tests performed in accordance with the test method given in the Manual of Tests and Criteria, part III, 33.4.1:
spontaneous ignition takes place in any step of the test procedure; or
there is an evolution of a flammable gas at a rate greater than 1 litre per kilogram of the substance per hour.
- 2.4.4.3
- Assignment of packing groups
- 2.4.4.3.1
- Packing group I shall be assigned to any substance which reacts vigorously with water at ambient temperatures and demonstrates generally a tendency for the gas produced to ignite spontaneously, or which reacts readily with water at ambient temperatures such that the rate of evolution of flammable gas is equal to or greater than 10 litres per kilogram of substance over any one minute.
- 2.4.4.3.2
- Packing group II shall be assigned to any substance which reacts readily with water at ambient temperatures such that the maximum rate of evolution of flammable gas is equal to or greater than 20 litres per kilogram of substance per hour, and which does not meet the criteria for packing group I.
- 2.4.4.3.3
- Packing group III shall be assigned to any substance which reacts slowly with water at ambient temperatures such that the maximum rate of evolution of flammable gas is greater than 1 litre per kilogram of substance per hour, and which does not meet the criteria for packing groups I or II.
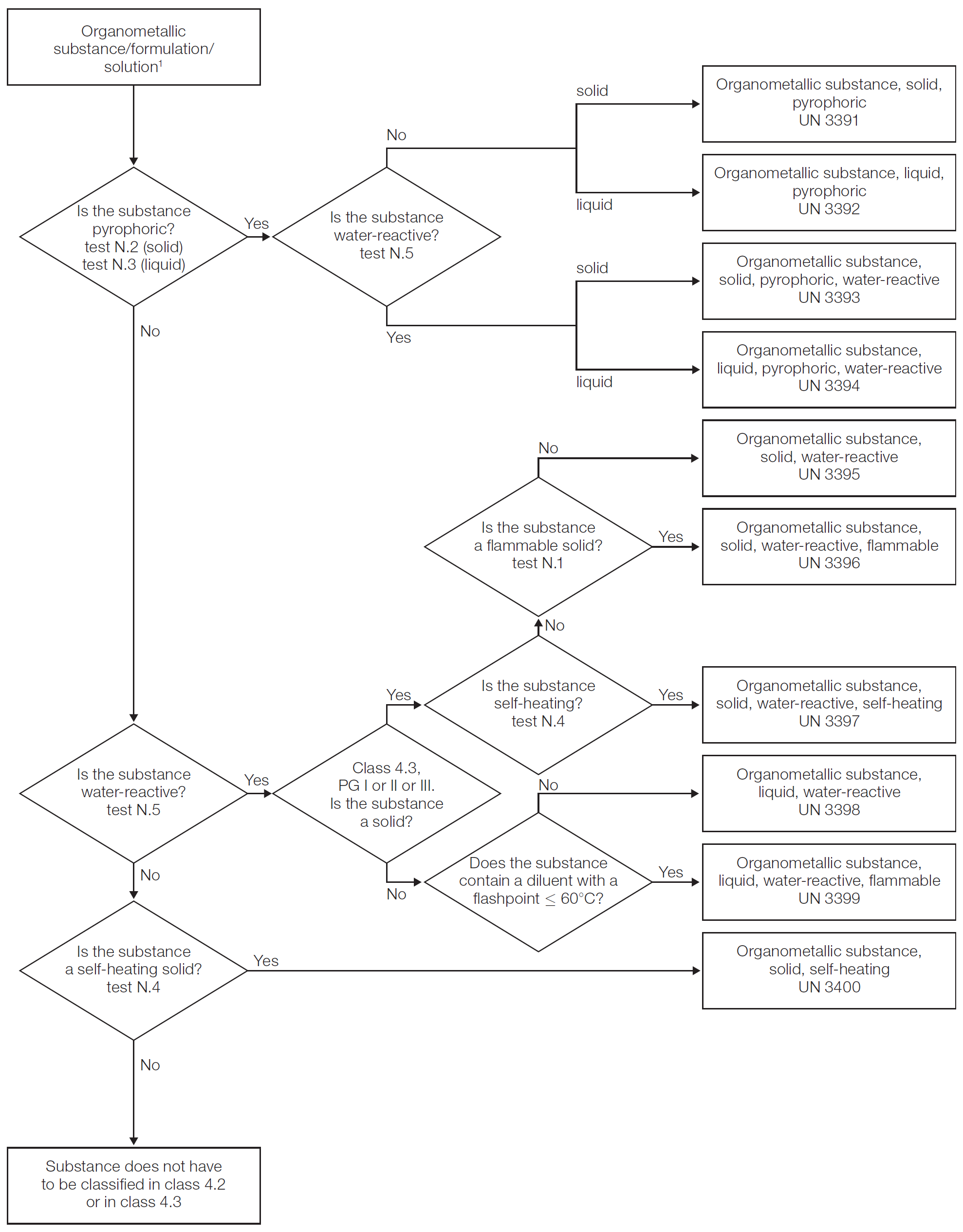
- 2.4.5
-
Classification of organometallic substances
Depending on their properties, organometallic substances may be classified in classes 4.2 or 4.3, as appropriate, in accordance with the following flowchart:
Flowchart scheme for organometallic substances1, 2