Chapter 2.6
Class 6 - Toxic and infectious substances
- 2.6.0
- Introductory notes
- Note 1:
- The word "toxic" has the same meaning as "poisonous".
- Note 2:
- Genetically modified microorganisms which do not meet the definition of a toxic or an infectious substance shall be considered for classification in class 9 and assigned to UN 3245.
- Note 3:
- Toxins from plant, animal or bacterial sources which do not contain any infectious substances, or toxins that are contained in substances which are not infectious substances, shall be considered for classification in class 6.1 and assigned to UN 3172 or UN 3462.
- 2.6.1
-
Definitions
Class 6 is subdivided into two classes as follows:
Class 6.1 - Toxic substances
These are substances liable either to cause death or serious injury or to harm human health if swallowed or inhaled, or by skin contact.Class 6.2 - Infectious substances
These are substances known or reasonably expected to contain pathogens. Pathogens are defined as microorganisms (including bacteria, viruses, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals. - 2.6.2
- Class 6.1 - Toxic substances
- 2.6.2.1
- Definitions and properties
- 2.6.2.1.1
- LD50 (median lethal dose) for acute oral toxicity is the statistically derived single dose of a substance that can be expected to cause death within 14 days in 50 per cent of young adult albino rats when administered by the oral route. The LD50 value is expressed in terms of mass of test substance per mass of test animal (mg/kg).
- 2.6.2.1.2
- LD50 for acute dermal toxicity is that dose of the substance which, administered by continuous contact for 24 hours with the bare skin of the albino rabbit, is most likely to cause death within 14 days in one half of the animals tested. The number of animals tested shall be sufficient to give a statistically significant result and be in conformity with good pharmacological practices. The result is expressed in milligrams per kilogram body mass.
- 2.6.2.1.3
- LC50 for acute toxicity on inhalation is that concentration of vapour, mist or dust which, administered by continuous inhalation to both male and female young adult albino rats for one hour, is most likely to cause death within 14 days in one half of the animals tested. A solid substance shall be tested if at least 10% (by mass) of its total mass is likely to be dust in the respirable range, such as the aerodynamic diameter of that particle fraction is 10 microns or less. A liquid substance shall be tested if a mist is likely to be generated in a leakage of the transport containment. For both solid and liquid substances, more than 90% (by mass) of a specimen prepared for inhalation toxicity testing shall be in the respirable range as defined above. The result is expressed in milligrams per litre of air for dusts and mists or in millilitres per cubic metre of air (parts per million) for vapours.
- 2.6.2.1.4
-
Properties
The dangers of poisoning which are inherent in these substances depend upon contact with the human body, that is by inhalation of vapours by unsuspecting persons at some distance from the cargo or the immediate dangers of physical contact with the substance. These have been considered in the context of the probability of accident occurring during transport by sea.
Nearly all toxic substances evolve toxic gases when involved in a fire or when heated to decomposition.
A substance specified as "stabilized" shall not be transported in an unstabilized condition.
- 2.6.2.2
- Assignment of packing groups to toxic substances
- 2.6.2.2.1
-
Toxic substances have for packing purposes been apportioned among packing groups according to the degree of their toxic hazards in transport:
Packing group I: substances and preparations presenting a high toxicity hazard;
Packing group II: substances and preparations presenting a medium toxicity hazard;
Packing group III: substances and preparations presenting a low toxicity hazard.
- 2.6.2.2.2
- In making this grouping, account has been taken of human experience in instances of accidental poisoning, and of special properties possessed by any individual substance, such as liquid state, high volatility, any special likelihood of penetration, and special biological effects.
- 2.6.2.2.3
-
In the absence of human experience, the grouping has been based on data obtained from animal experiments. Three possible routes of administration have been examined. These routes are exposure through:
oral ingestion;
dermal contact; and
inhalation of dusts, mists or vapours.
- 2.6.2.2.3.1
- For appropriate animal test data for the various routes of exposure, see 2.6.2.1. When a substance exhibits a different order of toxicity by two or more routes of administration, the highest degree of danger indicated by the tests has been used in assigning the packing group.
- 2.6.2.2.4
- The criteria to be applied for grouping a substance according to the toxicity it exhibits by all three routes of administration are presented in the following paragraphs.
- 2.6.2.2.4.1
-
The grouping criteria for the oral and dermal routes as well as for inhalation of dusts and mists are shown in the following table:
Grouping criteria for administration through oral ingestion, dermal contact and inhalation of dusts and mists Packing group Oral toxicity LD50 (mg/kg) Dermal toxicity LD50 (mg/kg) Inhalation toxicity by dusts and mists LC50 (mg/L) I ≤ 5.0 ≤ 50 ≤ 0.2 II > 5.0 and ≤50 > 50 and ≤200 > 0.2 and ≤2.0 III* > 50 and ≤300 > 200 and ≤1000 > 2.0 and ≤4.0 * Tear gas substances shall be included in packing group II even if their toxicity data correspond to packing group III values.
Note: Substances meeting the criteria of class 8 and with an inhalation toxicity of dusts and mists (LC50) leading to packing group I are only accepted for an allocation to class 6.1 if the toxicity through oral ingestion or dermal contact is at least in the range of packing group I or II. Otherwise an allocation to class 8 is made when appropriate (see 2.8.2.3).
- 2.6.2.2.4.2
- The criteria for inhalation toxicity of dusts and mists in 2.6.2.2.4.1 are based on LC50 data relating to one hour exposures, and where such information is available it shall be used. However, where only LC50 data relating to 4-hour exposures to dusts and mists are available, such figures can be multiplied by four and the product substituted in the above criteria, i.e. LC50 (4 hours) x 4 is considered the equivalent of LC50 (1 hour).
- 2.6.2.2.4.3
-
Liquids having toxic vapours shall be assigned to the following packing groups, where "V" is the saturated vapour concentration in mL/m³ air at 20°C and standard atmospheric pressure:
Packing group I: If V ≥ 10 LC50 and LC50 ≤ 1000 mL/m3.
Packing group II: If V ≥ LC50 and LC50 ≤ 3000 mL/m3, and do not meet the criteria for packing group I.
Packing group III: If V ≥ 1/5 LC50 and LC50 ≤ 5000 mL/m3, and do not meet the criteria for packing groups I or II.
Note: Tear gas substances shall be included in packing group II even if their toxicity data correspond to packing group III values.
- 2.6.2.2.4.4
-
In figure 2-3 the criteria according to 2.6.2.2.4.3 are expressed in graphical form, as an aid to easy classification. Because of approximations inherent in the use of graphs, substances falling on or near packing group borderlines shall be checked using numerical criteria.
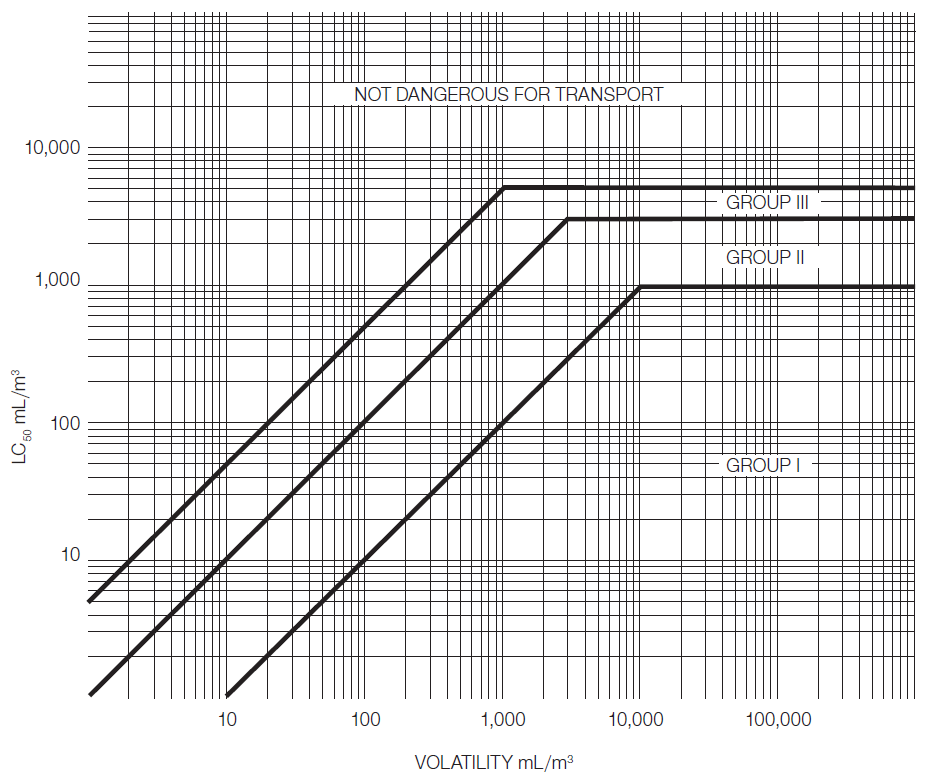
Figure 2-3 - Inhalation toxicity: packing group borderlines - 2.6.2.2.4.5
- The criteria for inhalation toxicity of vapours in 2.6.2.2.4.3 are based on LC50 data relating to one hour exposures, and where such information is available it shall be used. However, where only LC50 data relating to 4-hour exposures to the vapours are available, such figures can be multiplied by two and the product substituted in the above criteria, i.e. LC50 (4 hours) x 2 is considered the equivalent of LC50 (1 hour).
- 2.6.2.2.4.6
- Mixtures of liquids that are toxic by inhalation shall be assigned to packing groups according to 2.6.2.2.4.7 or 2.6.2.2.4.8.
- 2.6.2.2.4.7
-
If LC50 data are available for each of the toxic substances comprising a mixture, the packing group may be determined as follows:
Estimate the LC50 of the mixture using the formula:
\(LC_{50} \text{ (mixture)} = \frac{1}{\sum^{n}\limits_{i=1}\left(\frac{f_i}{LC_{50i}}\right)}\)
where:
- fi
- = mole fraction of the ith component substance of the mixture
- LC50i
- = mean lethal concentration of the ith component substance in mL/m3
Estimate the volatility of each component substance comprising the mixture using the formula:
\(V_i = \left(\frac{P_i \times 10^6}{101.3}\right) mL/m^3\)
where:
- Pi
- = the partial pressure of the ith component substance in kPa at 20°C and one atmosphere pressure.
Calculate the ratio of the volatility to the LC50 using the formula:
\(R = \sum^{n}\limits_{i=1}\left(\frac{V_i}{LC_{50i}}\right)\)
Using the calculated values of LC50 (mixture) and R, the packing group for the mixture is determined:
Packing group I: R ≥ 10 and LC50 (mixture) ≤ 1000 mL/m³.
Packing group II: R ≥ 1 and LC50 (mixture) ≤ 3000 mL/m³ and not meeting criteria for packing group I.
Packing group III: R ≥ 1/5 and LC50 (mixture) ≤ 5000 mL/m³ and not meeting criteria for packing groups I or II.
- 2.6.2.2.4.8
-
In the absence of LC50 data on the toxic constituent substances, the mixture may be assigned a packing group based on the following simplified threshold toxicity tests. When these threshold tests are used, the most restrictive packing group shall be determined and used for transporting the mixture.
A mixture is assigned to packing group I only if it meets both of the following criteria:
A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere of 1000 mL/m3 vaporized mixture in air. Ten albino rats (five male and five female) are exposed to the test atmosphere for one hour and observed for 14 days. If five or more of the animals die within the 14-day observation period, the mixture is presumed to have an LC50 equal to or less than 1000 mL/m3.
A sample of the vapour in equilibrium with the liquid mixture at 20°C is diluted with 9 equal volumes of air to form a test atmosphere. Ten albino rats (five male and five female) are exposed to the test atmosphere for one hour and observed for 14 days. If five or more of the animals die within the 14-day observation period, the mixture is presumed to have a volatility equal to or greater than 10 times the mixture LC50.
A mixture is assigned to packing group II only if it meets both of the following criteria, and the mixture does not meet the criteria for packing group I:
A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere of 3000 mL/m3 vaporized mixture in air. Ten albino rats (five male and five female) are exposed to the test atmosphere for one hour and observed for 14 days. If five or more of the animals die within the 14-day observation period, the mixture is presumed to have an LC50 equal to or less than 3000 mL/m3.
A sample of the vapour in equilibrium with the liquid mixture at 20°C is used to form a test atmosphere. Ten albino rats (five male and five female) are exposed to the test atmosphere for one hour and observed for 14 days. If five or more of the animals die within the 14-day observation period, the mixture is presumed to have a volatility equal to or greater than the mixture LC50.
A mixture is assigned to packing group III only if it meets both of the following criteria, and the mixture does not meet the criteria for packing groups I or II:
A sample of the liquid mixture is vaporized and diluted with air to create a test atmosphere of 5000 mL/m3 vaporized mixture in air. Ten albino rats (five male and five female) are exposed to the test atmosphere for one hour and observed for 14 days. If five or more of the animals die within the 14-day observation period, the mixture is presumed to have an LC50 equal to or less than 5000 mL/m3.
The vapour pressure of the liquid mixture is measured and if the vapour concentration is equal to or greater than 1000 mL/m3, the mixture is presumed to have a volatility equal to or greater than 1/5 the mixture LC50.
- 2.6.2.3
- Methods for determining oral and dermal toxicity of mixtures
- 2.6.2.3.1
- When classifying and assigning the appropriate packing group to mixtures in class 6.1, in accordance with the oral and dermal toxicity criteria in 2.6.2.2, it is necessary to determine the acute LD50 of the mixture.
- 2.6.2.3.2
-
If a mixture contains only one active substance, and the LD50 of that constituent is known, in the absence of reliable acute oral and dermal toxicity data on the actual mixture to be transported, the oral or dermal LD50 may be obtained by the following method:
\(LD_{50} \text{value of preparation} = \frac{LD_{50} \text{value of active substance} \times 100}{\text{percentage of active substance by mass}}\)
- 2.6.2.3.3
-
If a mixture contains more than one active constituent, there are three possible approaches that may be used to determine the oral or dermal LD50 of the mixture. The preferred method is to obtain reliable acute oral and dermal toxicity data on the actual mixture to be transported. If reliable, accurate data are not available, then either of the following methods may be performed:
Classify the formulation according to the most hazardous constituent of the mixture as if that constituent were present in the same concentration as the total concentration of all active constituents; or
Apply the formula:
\(\frac{C_A}{T_A} + \frac{C_B}{T_B} + \ldots \frac{C_Z}{T_Z} = \frac{100}{T_M}\)
where:
- C
- = the % concentration of constituent A, B ... Z in the mixture;
- T
- = the oral LD50 value of constituent A, B ... Z;
- TM
- = the oral LD50 value of the mixture.
Note: This formula can also be used for dermal toxicities provided that this information is available on the same species for all constituents. The use of this formula does not take into account any potentiation or protective phenomena.
- 2.6.2.4
- Classification of pesticides
- 2.6.2.4.1
- All active pesticide substances and their preparations for which the LC50 and/or LD50 values are known and which are classified in class 6.1 shall be classified under appropriate packing groups in accordance with the criteria given in 2.6.2.2. Substances and preparations which are characterized by subsidiary hazards shall be classified according to the precedence of hazard table in 2.0.3 with the assignment of appropriate packing groups.
- 2.6.2.4.2
-
If the oral or dermal LD50 value for a pesticide preparation is not known, but the LD50 value of its active substance(s) is known, the LD50 value for the preparation may be obtained by applying the procedures in 2.6.2.3.
Note: LD50 toxicity data for a number of common pesticides may be obtained from the most current edition of "The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification", available from the International Programme on Chemical Safety, World Health Organization (WHO), 1211 Geneva 27, Switzerland. While that publication may be used as a source of LD50 data for pesticides, its classification system shall not be used for purposes of transport classification of, or assignment of packing groups to, pesticides, which shall be in accordance with the provisions of this Code.
- 2.6.2.4.3
- The Proper Shipping Name used in the transport of the pesticide shall be selected from those referenced on the basis of the active ingredient, of the physical state of the pesticide and any subsidiary hazards which it may exhibit.
- 2.6.2.5
-
Substances not accepted for transport
Chemically unstable substances of class 6.1 shall not be accepted for transport unless the necessary precautions have been taken to prevent the possibility of a dangerous decomposition or polymerization under normal conditions of transport. For the precautions necessary to prevent polymerization, see special provision 386 of chapter 3.3. To this end particular care shall be taken to ensure that receptacles and tanks do not contain any substances liable to promote these reactions.
- 2.6.3
- Class 6.2 - Infectious substances
- 2.6.3.1
-
Definitions
For the purposes of this Code:
- 2.6.3.1.1
- Infectious substances are substances which are known or are reasonably expected to contain pathogens. Pathogens are defined as micro-organisms (including bacteria, viruses, parasites, fungi) and other agents such as prions, which can cause disease in humans or animals.
- 2.6.3.1.2
- Biological products are those products derived from living organisms which are manufactured and distributed in accordance with the requirements of appropriate national authorities, which may have special licensing requirements, and are used either for prevention, treatment, or diagnosis of disease in humans or animals, or for development, experimental or investigation purposes related thereto. They include, but are not limited to, finished or unfinished products such as vaccines.
- 2.6.3.1.3
- Cultures are the result of a process by which pathogens are intentionally propagated. This definition does not include human or animal patient specimens as defined in 2.6.3.1.4.
- 2.6.3.1.4
- Patient specimens are those collected directly from humans or animals, including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for purposes such as research, diagnosis, investigational activities, disease treatment and prevention.
- 2.6.3.1.5
- [Reserved]
- 2.6.3.1.6
- Medical or clinical wastes are wastes derived from the veterinary treatment of animals, the medical treatment of humans or from bio-research.
- 2.6.3.2
- Classification of infectious substances
- 2.6.3.2.1
- Infectious substances shall be classified in class 6.2 and assigned to UN 2814, UN 2900, UN 3291, UN 3373 or UN 3549, as appropriate.
- 2.6.3.2.2
- Infectious substances are divided into the following categories:
- 2.6.3.2.2.1
-
Category A: An infectious substance which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals. Indicative examples of substances that meet these criteria are given in the table in this paragraph.
Note: An exposure occurs when an infectious substance is released outside the protective packaging, resulting in physical contact with humans or animals.
Infectious substances meeting these criteria which cause disease in humans or in both humans and animals shall be assigned to UN 2814. Infectious substances which cause disease only in animals shall be assigned to UN 2900.
Assignment to UN 2814 or UN 2900 shall be based on the known medical history and symptoms of the source, human or animal, endemic local conditions, or professional judgement concerning individual circumstances of the human or animal source.
- Note 1:
- The Proper Shipping Name for UN 2814 is INFECTIOUS SUBSTANCE, AFFECTING HUMANS. The Proper Shipping Name for UN 2900 is INFECTIOUS SUBSTANCE, AFFECTING ANIMALS only.
- Note 2:
- The following table is not exhaustive. Infectious substances, including new or emerging pathogens, which do not appear in the table but which meet the same criteria shall be assigned to Category A. In addition, if there is doubt as to whether or not a substance meets the criteria it shall be included in Category A.
- Note 3:
-
In the following table, the microorganism names written in italics are bacteria, or fungi.
Indicative examples of infectious substances included in category A in any form unless otherwise indicated (2.6.3.2.2.1.1) UN Number and Proper Shipping Name Microorganism UN 2814
Infectious substance, affecting humansBacillus anthracis (cultures only) Brucella abortus (cultures only) Brucella melitensis (cultures only) Brucella suis (cultures only) Burkholderia mallei - Pseudomonas mallei - Glanders (cultures only) Burkholderia pseudomallei - Pseudomonas pseudomallei (cultures only) Chlamydia psittaci - avian strains (cultures only) Clostridium botulinum (cultures only) Coccidioides immitis (cultures only) Coxiella burnetii (cultures only) Crimean-Congo hemorrhagic fever virus Dengue virus (cultures only) Eastern equine encephalitis virus (cultures only) Escherichia coli, verotoxigenic (cultures only) Ebola virus Flexal virus Francisella tularensis (cultures only) Guanarito virus Hantaan virus Hantavirus causing hemorragic fever with renal syndrome Hendra virus Hepatitis B virus (cultures only) Herpes B virus (cultures only) Human immunodeficiency virus (cultures only) Highly pathogenic avian influenza virus (cultures only) Japanese Encephalitis virus (cultures only) Junin virus Kyasanur Forest disease virus Lassa virus Machupo virus Marburg virus Monkeypox virus Mycobacterium tuberculosis (cultures only) Nipah virus Omsk hemorrhagic fever virus Poliovirus (cultures only) Rabies virus (cultures only) Rickettsia prowazekii (cultures only) Rickettsia rickettsii (cultures only) Rift Valley fever virus (cultures only) Russian spring-summer encephalitis virus (cultures only) Sabia virus Shigella dysenteriae type 1 (cultures only) Tick-borne encephalitis virus (cultures only) Variola virus Venezuelan equine encephalitis virus (cultures only) West Nile virus (cultures only) Yellow fever virus (cultures only) Yersinia pestis (cultures only) UN 2900
Infectious substance, affecting animals onlyAfrican swine fever virus (cultures only) Avian paramyxovirus Type 1 - Velogenic Newcastle disease virus (cultures only) Classical swine fever virus (cultures only) Foot and mouth disease virus (cultures only) Lumpy skin disease virus (cultures only) Mycoplasma mycoides - Contagious bovine pleuropneumonia (cultures only) Peste des petits ruminants virus (cultures only) Rinderpest virus (cultures only) Sheep-pox virus (cultures only) Goatpox virus (cultures only) Swine vesicular disease virus (cultures only) Vesicular stomatitis virus (cultures only) - 2.6.3.2.2.2
-
Category B: An infectious substance which does not meet the criteria for inclusion in category A. Infectious substances in category B shall be assigned to UN 3373.
Note: The Proper Shipping Name for UN 3373 is BIOLOGICAL SUBSTANCE, CATEGORY B.
- 2.6.3.2.3
- Exemptions
- 2.6.3.2.3.1
- Substances which do not contain infectious substances or substances which are unlikely to cause disease in humans or animals are not subject to the provisions of this Code, unless they meet the criteria for inclusion in another class.
- 2.6.3.2.3.2
- Substances containing microorganisms which are non-pathogenic to humans or animals are not subject to the provisions of this Code unless they meet the criteria for inclusion in another class.
- 2.6.3.2.3.3
-
Substances in a form that any present pathogens have been neutralized or inactivated such that they no longer pose a health risk are not subject to the provisions of this Code unless they meet the criteria for inclusion in another class.
Note: Medical equipment which has been drained of free liquid is deemed to meet the requirements of this paragraph and is not subject to the provisions of this Code.
- 2.6.3.2.3.4
- Environmental samples (including food and water samples) which are not considered to pose a significant risk of infection are not subject to the provisions of this Code unless they meet the criteria for inclusion in another class.
- 2.6.3.2.3.5
- Dried blood spots, collected by applying a drop of blood onto absorbent material, are not subject to the provisions of this Code.
- 2.6.3.2.3.6
- Faecal occult blood screening samples are not subject to the provisions of this Code.
- 2.6.3.2.3.7
- Blood or blood components which have been collected for the purposes of transfusion or for the preparation of blood products to be used for transfusion or transplantation and any tissues or organs intended for use in transplantation as well as samples drawn in connection with such purposes are not subject to the provisions of this Code.
- 2.6.3.2.3.8
-
Human or animal specimens for which there is minimal likelihood that pathogens are present are not subject to the provisions of this Code if the specimen is transported in a packaging which will prevent any leakage and which is marked with the words “EXEMPT HUMAN SPECIMEN” or “EXEMPT ANIMAL SPECIMEN”, as appropriate. The packaging should meet the following conditions:
The packaging should consist of three components:
a leak-proof primary receptacle(s);
a leak-proof secondary packaging; and
an outer packaging of adequate strength for its capacity, mass and intended use, and with at least one surface having minimum dimensions of 100 mm x 100 mm.
For liquids, absorbent material in sufficient quantity to absorb the entire contents should be placed between the primary receptacle(s) and the secondary packaging so that, during transport, any release or leak of a liquid substance will not reach the outer packaging and will not compromise the integrity of the cushioning material.
When multiple fragile primary receptacles are placed in a single secondary packaging, they should be either individually wrapped or separated to prevent contact between them.
Note: An element of professional judgement is required to determine if a substance is exempt under this paragraph. That judgement should be based on the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions. Examples of specimens which may be transported under this paragraph include the blood or urine tests to monitor cholesterol levels, blood glucose levels, hormone levels, or prostate specific antibodies (PSA); those required to monitor organ function such as heart, liver or kidney function for humans or animals with non-infectious diseases, or therapeutic drug monitoring; those conducted for insurance or employment purposes and are intended to determine the presence of drugs or alcohol; pregnancy test; biopsies to detect cancer; and antibody detection in humans or animals in the absence of any concern for infection (e.g. evaluation of vaccine-induced immunity, diagnosis of autoimmune disease, etc.).
- 2.6.3.2.3.9
-
Except for:
medical devices or equipment contaminated with or containing infectious substances in category A (UN 2814 or UN 2900); and
medical devices or equipment contaminated with or containing other dangerous goods that meet the definition of another hazard class,
medical devices or equipment potentially contaminated with or containing infectious substances which are being transported for disinfection, cleaning, sterilization, repair, or equipment evaluation are not subject to the provisions of this Code if packed in packagings designed and constructed in such a way that, under normal conditions of transport, they cannot break, be punctured or leak their contents. Packagings shall be designed to meet the construction requirements listed in 6.1.4 or 6.6.4.
These packagings shall meet the general packing requirements of 4.1.1.1 and 4.1.1.2 and be capable of retaining the medical devices and equipment when dropped from a height of 1.2m.
The packagings shall be marked "USED MEDICAL DEVICE" or "USED MEDICAL EQUIPMENT". When using overpacks or unit loads these shall be marked in the same way, except when the inscription remains visible.
- 2.6.3.3
- Biological products
- 2.6.3.3.1
-
For the purposes of this Code, biological products are divided into the following groups:
those which are manufactured and packaged in accordance with the requirements of appropriate national authorities and transported for the purposes of final packaging or distribution, and use for personal health care by medical professionals or individuals. Substances in this group are not subject to the provisions of this Code;
those which do not fall under .1 and are known or reasonably believed to contain infectious substances and which meet the criteria for inclusion in category A or category B. Substances in this group shall be assigned to UN 2814, UN 2900 or UN 3373, as appropriate.
Note: Some licensed biological products may present a biohazard only in certain parts of the world. Competent authorities may require that such biological products comply with local requirements for infectious substances or may impose other restrictions.
- 2.6.3.4
- Genetically modified microorganisms and organisms
- 2.6.3.4.1
- Genetically modified microorganisms not meeting the definition of infectious substance shall be classified in accordance with chapter 2.9.
- 2.6.3.5
- Medical or clinical wastes
- 2.6.3.5.1
-
Medical or clinical wastes containing:
- Category A infectious substances shall be assigned to UN 2814, UN 2900 or UN 3549 as appropriate. Solid medical waste containing Category A infectious substances generated from the medical treatment of humans or veterinary treatment of animals may be assigned to UN 3549. The UN 3549 entry shall not be used for waste from bio-research or liquid waste;
- Category B infectious substances shall be assigned to UN 3291.
- 2.6.3.5.2
-
Medical or clinical wastes which are reasonably believed to have a low probability of containing infectious substances shall be assigned to UN 3291. For the assignment, international, regional or national waste catalogues may be taken into account.
Note: The Proper Shipping Name for UN 3291 is CLINICAL WASTE, UNSPECIFIED, N.O.S. or (BIO) MEDICAL WASTE, N.O.S. or REGULATED MEDICAL WASTE, N.O.S.
- 2.6.3.5.3
- Decontaminated medical or clinical wastes which previously contained infectious substances are not subject to the provisions of this Code unless they meet the criteria for inclusion in another class.
- 2.6.3.6
- Infected animals
- 2.6.3.6.1
- Unless an infectious substance cannot be consigned by any other means, live animals shall not be used to consign such a substance. A live animal which has been intentionally infected and is known or suspected to contain an infectious substance shall only be transported under terms and conditions approved by the competent authority.