Chapter 2.9
Miscellaneous dangerous substances and articles (class 9) and environmentally hazardous substances
- Note 1:
- For the purposes of this Code, the environmentally hazardous substances (aquatic environment) criteria contained in this chapter apply to the classification of marine pollutants (see 2.10).
- Note 2:
- Although the environmentally hazardous substances (aquatic environment) criteria apply to all hazard classes, except for class 7 (see paragraphs 2.10.2.3, 2.10.2.5 and 2.10.3.2), the criteria have been included in this chapter.
- 2.9.1
- Definitions
- 2.9.1.1
- Class 9 substances and articles (miscellaneous dangerous substances and articles) are substances and articles which, during transport, present a danger not covered by other classes.
- 2.9.2
- Assignment to class 9
- 2.9.2.1
-
Class 9 includes, inter alia:
substances and articles not covered by other classes which experience has shown, or may show, to be of such a dangerous character that the provisions of part A of chapter VII of SOLAS, as amended, shall apply.
substances not subject to the provisions of part A in chapter VII of the aforementioned Convention, but to which the provisions of Annex III of MARPOL, as amended, apply.
- 2.9.2.2
-
The substances and articles of class 9 are subdivided as follows:
Substances which, on inhalation as fine dust, may endanger health
- 2212
- ASBESTOS, AMPHIBOLE (amosite, tremolite, actinolite, anthophyllite, crocidolite)
- 2590
- ASBESTOS, CHRYSOTILE
Substances evolving flammable vapour
- 2211
- POLYMERIC BEADS, EXPANDABLE, evolving flammable vapour
- 3314
- PLASTICS MOULDING COMPOUND in dough, sheet or extruded rope form evolving flammable vapour
Lithium batteries
- 3090
- LITHIUM METAL BATTERIES (including lithium alloy batteries)
- 3091
- LITHIUM METAL BATTERIES CONTAINED IN EQUIPMENT (including lithium alloy batteries) or
- 3091
- LITHIUM METAL BATTERIES PACKED WITH EQUIPMENT (including lithium alloy batteries)
- 3480
- LITHIUM ION BATTERIES (including lithium ion polymer batteries)
- 3481
- LITHIUM ION BATTERIES CONTAINED IN EQUIPMENT (including lithium ion polymer batteries) or
- 3481
- LITHIUM ION BATTERIES PACKED WITH EQUIPMENT (including lithium ion polymer batteries)
- 3536
- LITHIUM BATTERIES INSTALLED IN CARGO TRANSPORT UNIT (lithium ion batteries or lithium metal batteries)
Note: See 2.9.4.
Capacitors
- 3499
- CAPACITOR, ELECTRIC DOUBLE LAYER (with an energy storage capacity greater than 0.3Wh)
- 3508
- CAPACITOR, ASYMMETRIC (with an energy storage capacity greater than 0.3Wh).
Life-saving appliances
- 2990
- LIFE-SAVING APPLIANCES, SELF-INFLATING
- 3072
- LIFE-SAVING APPLIANCES NOT SELF-INFLATING containing dangerous goods as equipment
- 3268
- SAFETY DEVICES, electrically initiated
Substances and articles which, in the event of fire, may form dioxins
This group of substances includes:
- 2315
- POLYCHLORINATED BIPHENYLS, LIQUID
- 3432
- POLYCHLORINATED BIPHENYLS, SOLID
- 3151
- POLYHALOGENATED BIPHENYLS, LIQUID or
- 3151
- HALOGENATED MONOMETHYLDIPHENYLMETHANES, LIQUID or
- 3151
- POLYHALOGENATED TERPHENYLS, LIQUID
- 3152
- POLYHALOGENATED BIPHENYLS, SOLID or
- 3152
- HALOGENATED MONOMETHYLDIPHENYLMETHANES, SOLID or
- 3152
- POLYHALOGENATED TERPHENYLS, SOLID
Examples of articles are transformers, condensers and apparatus containing those substances.
Substances transported or offered for transport at elevated temperatures
- 3257
- ELEVATED TEMPERATURE LIQUID, N.O.S., at or above 100ºC and below its flashpoint (including molten metal, molten salts, etc.)
- 3258
- ELEVATED TEMPERATURE SOLID, N.O.S., at or above 240ºC
Environmentally hazardous substances
- 3077
- ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.
- 3082
- ENVIRONMENTALLY HAZARDOUS SUBSTANCE, LIQUID, N.O.S.
These entries are used for substances and mixtures which are dangerous to the aquatic environment that do not meet the classification criteria of any other class or another substance within class 9. These entries may also be used for wastes not otherwise subject to the provisions of this Code but which are covered under the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal and for substances designated to be environmentally hazardous substances by the competent authority of the country of origin, transit or destination which do not meet the criteria for an environmentally hazardous substance according to the provisions of this Code or for any other hazard class. The criteria for substances which are hazardous to the aquatic environment are given in section 2.9.3.
Genetically modified microorganisms (GMMOs) and genetically modified organisms (GMOs)
GMMOs and GMOs which do not meet the definition of toxic substances (see 2.6.2) or infectious substances (see 2.6.3) shall be assigned to UN 3245.
GMMOs or GMOs are not subject to the provisions of this Code when authorized for use by the competent authorities of the countries of origin, transit and destination.
Genetically modified live animals shall be transported under terms and conditions of the competent authorities of the countries of origin and destination.
Ammonium nitrate based fertilizers
- 2071
- AMMONIUM NITRATE BASED FERTILIZER
Solid ammonium nitrate based fertilizers shall be classified in accordance with the procedure as set out in the Manual of Tests and Criteria, part III, section 39.
Other substances or articles presenting a danger during transport, but not meeting the definitions of another class:
- 1841
- ACETALDEHYDE AMMONIA
- 1845
- CARBON DIOXIDE, SOLID (DRY ICE)
- 1931
- ZINC DITHIONITE (ZINC HYDROSULPHITE)
- 1941
- DIBROMODIFLUOROMETHANE
- 1990
- BENZALDEHYDE
- 2216
- FISH MEAL (FISH SCRAP), STABILIZED
- 2807
- MAGNETIZED MATERIAL
- 2969
- CASTOR BEANS or
- 2969
- CASTOR MEAL or
- 2969
- CASTOR POMACE or
- 2969
- CASTOR FLAKE
- 3166
- VEHICLE, FLAMMABLE GAS POWERED or
- 3166
- VEHICLE, FLAMMABLE LIQUID POWERED or
- 3166
- VEHICLE, FUEL CELL, FLAMMABLE GAS POWERED or
- 3166
- VEHICLE, FUEL CELL, FLAMMABLE LIQUID POWERED
- 3171
- BATTERY-POWERED VEHICLE or
- 3171
- BATTERY-POWERED EQUIPMENT
- 3316
- CHEMICAL KIT or
- 3316
- FIRST AID KIT
- 3334
- AVIATION REGULATED LIQUID, N.O.S.
- 3335
- AVIATION REGULATED SOLID, N.O.S.
- 3359
- FUMIGATED CARGO TRANSPORT UNIT
- 3363
- DANGEROUS GOODS IN ARTICLES or
- 3363
- DANGEROUS GOODS IN MACHINERY or
- 3363
- DANGEROUS GOODS IN APPARATUS
- 3496
- BATTERIES, NICKEL-METAL HYDRIDE
- 3509
- PACKAGINGS, DISCARDED, EMPTY, UNCLEANED
- 3530
- ENGINE, INTERNAL COMBUSTION or
- 3530
- MACHINERY, INTERNAL COMBUSTION
- 3548
- ARTICLES CONTAINING MISCELLANEOUS DANGEROUS GOODS N.O.S.
- 2.9.3
- Environmentally hazardous substances (aquatic environment)
- 2.9.3.1
- General definitions
- 2.9.3.1.1
-
Environmentally hazardous substances include, inter alia, liquid or solid substances pollutant to the aquatic environment and solutions and mixtures of such substances (such as preparations and wastes).
For the purposes of this section,
Substance means chemical elements and their compounds in the natural state or obtained by any production process, including any additive necessary to preserve the stability of the product and any impurities deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition.
- 2.9.3.1.2
- The aquatic environment may be considered in terms of the aquatic organisms that live in the water, and the aquatic ecosystem of which they are part1. The basis, therefore, of the identification of hazard is the aquatic toxicity of the substance or mixture, although this may be modified by further information on the degradation and bioaccumulation behaviour.
- 2.9.3.1.3
- While the following classification procedure is intended to apply to all substances and mixtures, it is recognized that in some cases, e.g. metals or poorly soluble inorganic compounds, special guidance will be necessary2.
- 2.9.3.1.4
-
The following definitions apply for acronyms or terms used in this section:
- BCF
- bioconcentration Factor;
- BOD
- biochemical Oxygen Demand;
- COD
- chemical Oxygen Demand;
- GLP
- good Laboratory Practices;
- ECx
- the concentration associated with x% response;
- EC50
- the effective concentration of substance that causes 50% of the maximum response;
- ErC50
- EC50 in terms of reduction of growth;
- Kow
- octanol/water partition coefficient;
- LC50 (50% lethal concentration)
- the concentration of a substance in water which causes the death of 50% (one half) in a group of test animals;
- L(E)C50
- LC50 or EC50;
- NOEC (no observed effect concentration)
- the test concentration immediately below the lowest tested concentration with statistically significant adverse effect. The NOEC has no statistically significant adverse effect compared to the control;
- OECD Test Guidelines
- Test guidelines published by the Organization for Economic Co-operation and Development (OECD).
- 2.9.3.2
- Definitions and data requirements
- 2.9.3.2.1
-
The basic elements for classification of environmentally hazardous substances (aquatic environment) are:
acute aquatic toxicity;
chronic aquatic toxicity;
potential for or actual bioaccumulation; and
degradation (biotic or abiotic) for organic chemicals.
- 2.9.3.2.2
- While data from internationally harmonized test methods are preferred, in practice, data from national methods may also be used where they are considered as equivalent. In general, it has been agreed that freshwater and marine species toxicity data can be considered as equivalent data and are preferably to be derived using OECD Test Guidelines or equivalent according to the principles of good laboratory practices (GLP). Where such data are not available, classification shall be based on the best available data.
- 2.9.3.2.3
-
Acute aquatic toxicity means the intrinsic property of a substance to be injurious to an organism in a short-term aquatic exposure to that substance.
Acute (short-term) hazard, for classification purposes, means the hazard of a chemical caused by its acute toxicity to an organism during short-term aquatic exposure to that chemical.
Acute aquatic toxicity shall normally be determined using a fish 96 h LC50 (OECD Test Guideline 203 or equivalent), a crustacea species 48 h EC50 (OECD Test Guideline 202 or equivalent) and/or an algal species 72 or 96 h EC50 (OECD Test Guideline 201 or equivalent). These species are considered as surrogate for all aquatic organisms and data on other species such as Lemna may also be considered if the test methodology is suitable.
- 2.9.3.2.4
-
Chronic aquatic toxicity means the intrinsic property of a substance to cause adverse effects to aquatic organisms during aquatic exposures which are determined in relation to the life cycle of the organism.
Long-term hazard, for classification purposes, means the hazard of a chemical caused by its chronic toxicity following long-term exposure in the aquatic environment.
Chronic toxicity data are less available than acute data and the range of testing procedures less standardized. Data generated according to the OECD Test Guidelines 210 (Fish Early Life Stage) or 211 (Daphnia Reproduction) and 201 (Algal Growth Inhibition) may be accepted. Other validated and internationally accepted tests may also be used. The NOECs or other equivalent ECx shall be used.
- 2.9.3.2.5
-
Bioaccumulation means net result of uptake, transformation and elimination of a substance in an organism due to all routes of exposure (i.e. air, water, sediment/soil and food).
The potential for bioaccumulation shall normally be determined by using the octanol/water partition coefficient, usually reported as a log Kow determined according to OECD Test Guidelines 107, 117 or 123. While this represents a potential to bioaccumulate, an experimentally determined bioconcentration factor (BCF) provides a better measure and shall be used in preference when available. A BCF shall be determined according to OECD Test Guideline 305.
- 2.9.3.2.6
-
Degradation means the decomposition of organic molecules to smaller molecules and eventually to carbon dioxide, water and salts.
Environmental degradation may be biotic or abiotic (e.g. hydrolysis) and the criteria used reflect this fact. Ready biodegradation is most easily defined using the biodegradability tests (A to F) of OECD Test Guidelines 301. A pass level in these tests may be considered as indicative of rapid degradation in most environments. These are freshwater tests and thus the use of the results from OECD Test Guideline 306, which is more suitable for marine environments, has also been included. Where such data are not available, a BOD(5 days)/COD ratio ≥ 0.5 is considered as indicative of rapid degradation. Abiotic degradation such as hydrolysis, primary degradation, both abiotic and biotic, degradation in non-aquatic media and proven rapid degradation in the environment may all be considered in defining rapid degradability.³
³Special guidance on data interpretation is provided in chapter 4.1 and annex 9 of the GHS.
Substances are considered rapidly degradable in the environment if the following criteria are met:
In 28-day ready biodegradation studies, the following levels of degradation are achieved:
tests based on dissolved organic carbon: 70%;
tests based on oxygen depletion or carbon dioxide generation: 60% of theoretical maxima.
These levels of biodegradation shall be achieved within 10 days of the start of degradation which point is taken as the time when 10% of the substance has been degraded, unless the substance is identified as a complex, multi-component substance with structurally similar constituents. In this case, and where there is sufficient justification, the 10-day window condition may be waived and the pass level applied at 28 days;3
in those cases where only BOD and COD data are available, when the ratio of BOD5/COD is ≥ 0.5; or
if other convincing scientific evidence is available to demonstrate that the substance or mixture can be degraded (biotically and/or abiotically) in the aquatic environment to a level above 70% within a 28-day period.
- 2.9.3.3
- Substance classification categories and criteria
- 2.9.3.3.1
-
Substances shall be classified as "environmentally hazardous substances (aquatic environment)", if they satisfy the criteria for Acute 1, Chronic 1 or Chronic 2, according to table 2.9.1. These criteria describe in detail the classification categories. They are diagrammatically summarized in table 2.9.2.
Table 2.9.1 - Categories for substances hazardous to the aquatic environment (see note 1) (a) Acute (short-term) aquatic hazard
Category: Acute 1 (see note 2) 96 hr LC50 (for fish) ≤ 1 mg/L and/or 48 hr EC50 (for crustacea) ≤ 1 mg/L and/or 72 or 96 hr ErC50 (for algae or other aquatic plants) ≤ 1 mg/L (see note 3) (b) Long-term aquatic hazard (see also figure 2.9.1)
(i) Non-rapidly degradable substances (see note 4) for which there are adequate chronic toxicity data available
Category: Chronic 1 (see note 2) Chronic NOEC or ECx (for fish) ≤ 0.1 mg/L and/or Chronic NOEC or ECx (for crustacea) ≤ 0.1 mg/L and/or Chronic NOEC or ECx (for algae or other aquatic plants) ≤ 0.1 mg/L Category: Chronic 2 Chronic NOEC or ECx (for fish) ≤ 1 mg/L and/or Chronic NOEC or ECx (for crustacea) ≤ 1 mg/L and/or Chronic NOEC or ECx (for algae or other aquatic plants) ≤ 1 mg/L (ii) Rapidly degradable substances for which there are adequate chronic toxicity data available
Category: Chronic 1 (see note 2) Chronic NOEC or ECx (for fish) ≤ 0.01 mg/L and/or Chronic NOEC or ECx (for crustacea) ≤ 0.01 mg/L and/or Chronic NOEC or ECx (for algae or other aquatic plants) ≤ 0.01 mg/L Category: Chronic 2 Chronic NOEC or ECx (for fish) ≤ 0.1 mg/L and/or Chronic NOEC or ECx (for crustacea) ≤ 0.1 mg/L and/or Chronic NOEC or ECx (for algae or other aquatic plants) ≤ 0.1 mg/L (iii) Substances for which adequate chronic toxicity data are not available
Category: Chronic 1 (see note 2) 96 hr LC50 (for fish) ≤ 1 mg/L and/or 48 hr EC50 (for crustacea) ≤ 1 mg/L and/or 72 or 96 hr ErC50 (for algae or other aquatic plants) ≤ 1 mg/L (see note 3) and the substance is not rapidly degradable and/or the experimentally determined BCF is ≥ 500 (or, if absent, the log Kow ≥ 4) (see notes 4 and 5) Category: Chronic 2 96 hr LC50 (for fish) > 1 but ≤ 10 mg/L and/or 48 hr EC50 (for crustacea) > 1 but ≤ 10 mg/L and/or 72 or 96 hr ErC50 (for algae or other aquatic plants) > 1 but ≤ 10 mg/L and the substance is not rapidly degradable and/or the experimentally determined BCF is ≥ 500 (or, if absent, the log Kow ≥ 4) (see notes 4 and 5 Note 1: The organisms fish, crustacea and algae are tested as surrogate species covering a range of trophic levels and taxa, and the test methods are highly standardized. Data on other organisms may also be considered, however, provided they represent equivalent species and test endpoints.
Note 2: When classifying substances as Acute 1 and/or Chronic 1 it is necessary at the same time to indicate an appropriate M factor (see 2.9.3.4.6.4) to apply the summation method.
Note 3: Where the algal toxicity ErC50 (= EC50 (growth rate)) falls more than 100 times below the next most sensitive species and results in a classification based solely on this effect, consideration shall be given to whether this toxicity is representative of the toxicity to aquatic plants. Where it can be shown that this is not the case, professional judgment shall be used in deciding if classification shall be applied. Classification shall be based on the ErC50. In circumstances where the basis of the EC50 is not specified and no ErC50 is recorded, classification shall be based on the lowest EC50 available.
Note 4: Lack of rapid degradability is based on either a lack of ready biodegradability or other evidence of lack of rapid degradation. When no useful data on degradability are available, either experimentally determined or estimated data, the substance shall be regarded as not rapidly degradable.
Note 5: Potential to bioaccumulate, based on an experimentally derived BCF ≥ 500 or, if absent, a log Kow ≥ 4 provided log Kow is an appropriate descriptor for the bioaccumulation potential of the substance. Measured log Kow values take precedence over estimated values and measured BCF values take precedence over log Kow values.
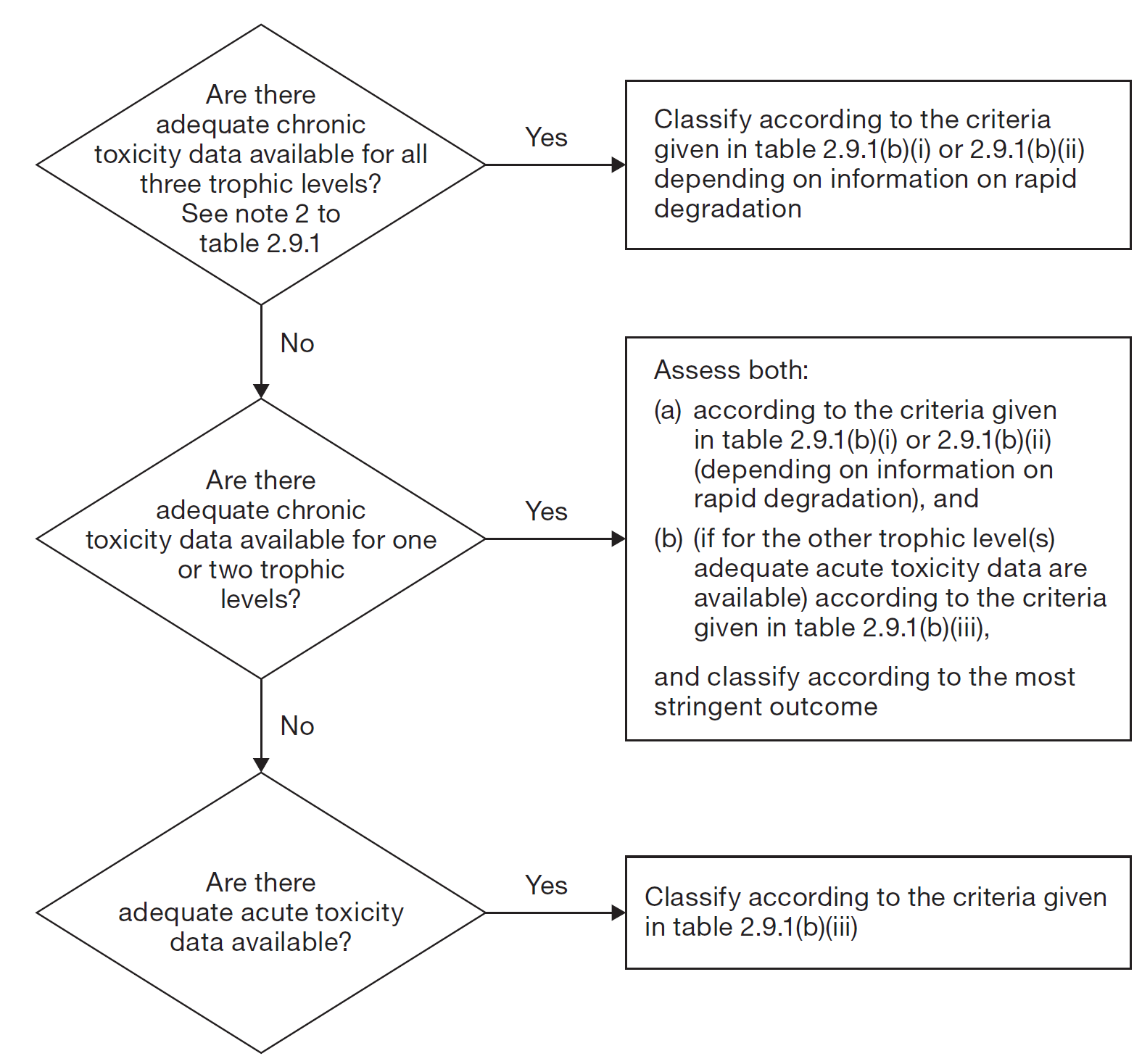
Figure 2.9.1 - Categories for substances long-term hazardous to the aquatic environment - 2.9.3.3.2
-
The classification scheme in table 2.9.2 below summarizes the classification criteria for substances.
Table 2.9.2 - Classification scheme for substances hazardous to the aquatic environment Classification categories Acute Hazard
(see note 1)Long-term hazard
(see note 2)Adequate chronic toxicity data available Adequate chronic toxicity data not available
(see note 1)Non-rapidly degradable substances
(see note 3)Rapidly degradable substances
(see note 3)Category: Acute 1 Category: Chronic 1 Category Chronic 1 Category: Chronic 1 L(E)C50 ≤ 1.00 NOEC or ECx ≤ 0.1 NOEC or ECx ≤ 0.01 L(E)C50 ≤ 1.00 and lack of rapid degradability and/or BCF ≥ 500 or, if absent, log Kow ≥ 4 Category: Chronic 2 Category: Chronic 2 Category: Chronic 2 0.1 < NOEC or ECx ≤ 1 0.01 < NOEC or ECx ≤ 0.1 1.00 < L(E)C50 ≤ 10.0 and lack of rapid degradability and/or BCF ≥ 500 or, if absent, log Kow ≥ 4 Note 1: Acute toxicity band based on L(E)C50 values in mg/L for fish, crustacea and/or algae or other aquatic plants (or Quantitative Structure Activity Relationships (QSAR) estimation if no experimental data4).
Note 2: Substances are classified in the various chronic categories unless there are adequate chronic toxicity data available for all three trophic levels above the water solubility or above 1 mg/L. ("Adequate" means that the data sufficiently cover the endpoint of concern. Generally this would mean measured test data, but in order to avoid unnecessary testing it can on a case by case basis also be estimated data, e.g. (Q)SAR, or for obvious cases expert judgment).
Note 3: Chronic toxicity band based on NOEC or equivalent ECx values in mg/L for fish or crustacea or other recognized measures for chronic toxicity.
- 2.9.3.4
- Mixtures classification categories and criteria
- 2.9.3.4.1
- The classification system for mixtures covers the classification categories which are used for substances, meaning categories Acute 1 and Chronic 1 and 2. In order to make use of all available data for purposes of classifying the aquatic environmental hazards of the mixture, the following assumption is made and is applied where appropriate: The "relevant ingredients" of a mixture are those which are present in a concentration equal to or greater than 0.1% (by mass) for ingredients classified as Acute and/or Chronic 1 and equal to or greater than 1% for other ingredients, unless there is a presumption (e.g., in the case of highly toxic ingredients) that an ingredient present at less than 0.1% can still be relevant for classifying the mixture for aquatic environmental hazards.
- 2.9.3.4.2
-
The approach for classification of aquatic environmental hazards is tiered, and is dependent upon the type of information available for the mixture itself and for its ingredients. Elements of the tiered approach include:
classification based on tested mixtures;
classification based on bridging principles;
the use of "summation of classified ingredients" and/or an "additivity formula".
Figure 2.9.2 outlines the process to be followed.
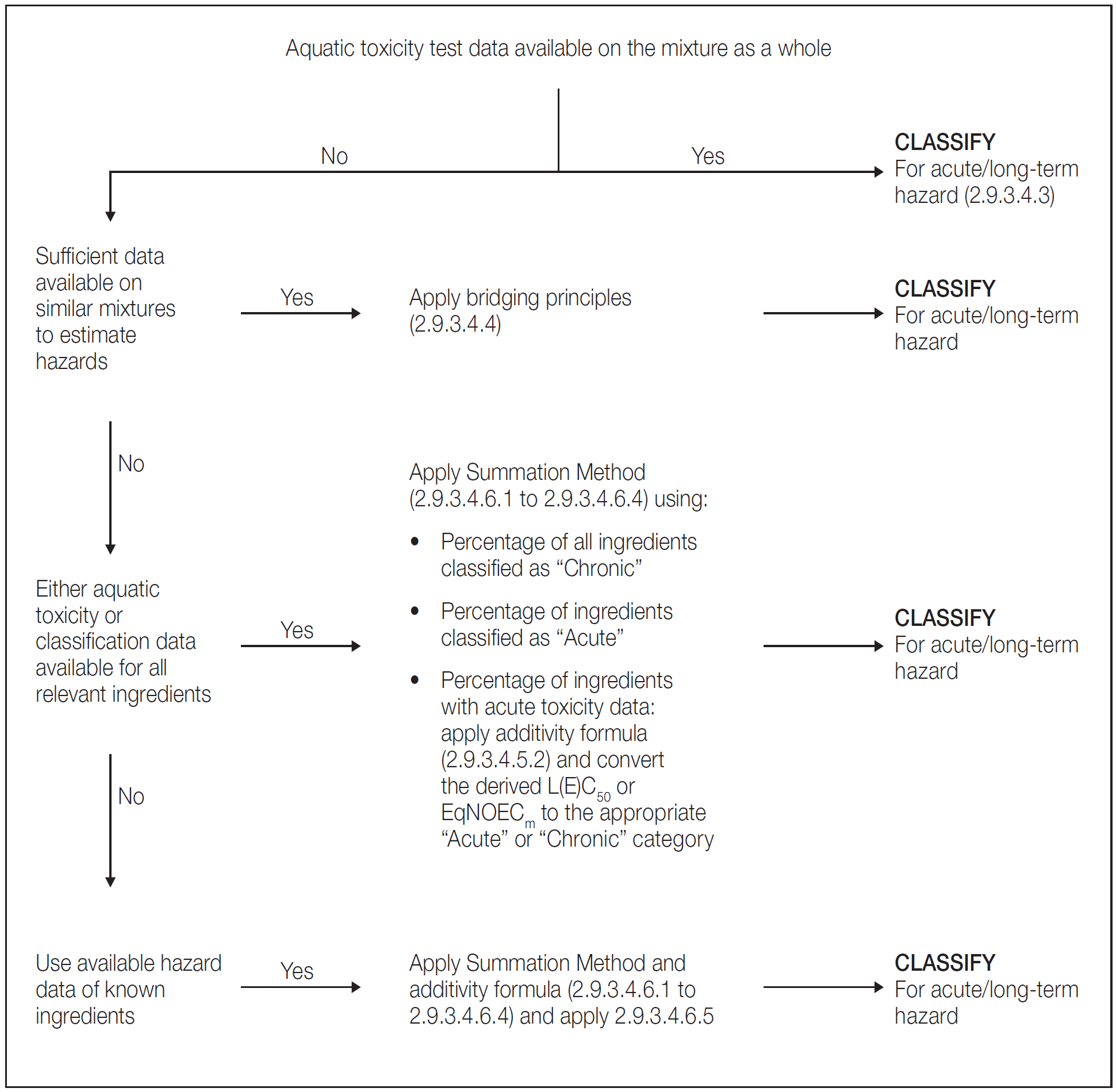
Figure 2.9.2 - Tiered approach to classification of mixtures for acute and long-term aquatic environmental hazards - 2.9.3.4.3
- Classification of mixtures when toxicity data are available for the complete mixture
- 2.9.3.4.3.1
- When the mixture as a whole has been tested to determine its aquatic toxicity, this information shall be used for classifying the mixture according to the criteria that have been agreed for substances. The classification is normally based on the data for fish, crustacea and algae/plants (see 2.9.3.2.3 and 2.9.3.2.4). When adequate acute or chronic data for the mixture as a whole are lacking, "bridging principles" or "summation method" shall be applied (see 2.9.3.4.4 to 2.9.3.4.6).
- 2.9.3.4.3.2
- The long-term hazard classification of mixtures requires additional information on degradability and in certain cases bioaccumulation. There are no degradability and bioaccumulation data for mixtures as a whole. Degradability and bioaccumulation tests for mixtures are not used as they are usually difficult to interpret, and such tests may be meaningful only for single substances.
- 2.9.3.4.3.3
-
Classification for category Acute 1
When there are adequate acute toxicity test data (LC50 or EC50) available for the mixture as a whole showing L(E)C50 ≤ 1 mg/L:
- Classify the mixture as Acute 1 in accordance with table 2.9.1 (a);
When there are acute toxicity test data (LC50(s) or EC50(s) available for the mixture as a whole showing L(E)C50(s) > 1 mg/L, or above the water solubility:
- No need to classify for acute hazard under this Code.
- 2.9.3.4.3.4
-
Classification for categories Chronic 1 and 2
When there are adequate chronic toxicity data (ECx or NOEC) available for the mixture as a whole showing ECx or NOEC of the tested mixture ≤ 1 mg/L:
- classify the mixture as Chronic 1 or 2 in accordance with table 2.9.1 (b) (ii) (rapidly degradable) if the available information allows the conclusion that all relevant ingredients of the mixture are rapidly degradable;
ii. classify the mixture as Chronic 1 or 2 in all other cases in accordance with [table 2.9.1]() (b) (i) (non-rapidly degradable);When there are adequate chronic toxicity data (ECx or NOEC) available for the mixture as a whole showing ECx(s) or NOEC(s) of the tested mixture > 1 mg/L or above the water solubility:
- No need to classify for long-term hazard under this Code.
- 2.9.3.4.4
- Classification of mixtures when toxicity data are not available for the complete mixture: bridging principles
- 2.9.3.4.4.1
- Where the mixture itself has not been tested to determine its aquatic environmental hazard, but there are sufficient data on the individual ingredients and similar tested mixtures to adequately characterize the hazards of the mixture, these data shall be used in accordance with the following agreed bridging rules. This ensures that the classification process uses the available data to the greatest extent possible in characterizing the hazards of the mixture without the necessity for additional testing in animals.
- 2.9.3.4.4.2
- Dilution
- 2.9.3.4.4.2.1
- Where a new mixture is formed by diluting a tested mixture or a substance with a diluent which has an equivalent or lower aquatic hazard classification than the least toxic original ingredient and which is not expected to affect the aquatic hazards of other ingredients, then the resulting mixture shall be classified as equivalent to the original tested mixture or substance. Alternatively, the method explained in 2.9.3.4.5 may be applied.
- 2.9.3.4.4.2.2
- If a mixture is formed by diluting another classified mixture or a substance with water or other totally non-toxic material, the toxicity of the mixture shall be calculated from the original mixture or substance.
- 2.9.3.4.4.3
- Batching
- 2.9.3.4.4.3.1
- The aquatic hazard classification of a tested production batch of a mixture shall be assumed to be substantially equivalent to that of another untested production batch of the same commercial product when produced by or under the control of the same manufacturer, unless there is reason to believe there is significant variation such that the aquatic hazard classification of the untested batch has changed. If the latter occurs, new classification is necessary.
- 2.9.3.4.4.4
- Concentration of mixtures which are classified with the most severe classification categories (Chronic 1 and Acute 1)
- 2.9.3.4.4.4.1
- If a tested mixture is classified as Chronic 1 and/or Acute 1, and the ingredients of the mixture which are classified as Chronic 1 and/or Acute 1 are further concentrated, the more concentrated untested mixture shall be classified with the same classification category as the original tested mixture without additional testing.
- 2.9.3.4.4.5
- Interpolation within one toxicity category
- 2.9.3.4.4.5.1
- For three mixtures (A, B and C) with identical ingredients, where mixtures A and B have been tested and are in the same toxicity category, and where untested mixture C has the same toxicologically active ingredients as mixtures A and B but has concentrations of toxicologically active ingredients intermediate to the concentrations in mixtures A and B, then mixture C is assumed to be in the same category as A and B.
- 2.9.3.4.4.6
- Substantially similar mixtures
- 2.9.3.4.4.6.1
-
Given the following:
Two mixtures:
- A + B
- C + B
The concentration of ingredient B is essentially the same in both mixtures;
The concentration of ingredient A in mixture (i) equals that of ingredient C in mixture (ii);
Data on aquatic hazards for A and C are available and are substantially equivalent, i.e. they are in the same hazard category and are not expected to affect the aquatic toxicity of B.
If mixture (i) or (ii) is already classified based on test data, then the other mixture can be assigned the same hazard category.
- 2.9.3.4.5
- Classification of mixtures when toxicity data are available for all ingredients or only for some ingredients of the mixture
- 2.9.3.4.5.1
- The classification of a mixture shall be based on summation of the concentrations of its classified ingredients. The percentage of ingredients classified as Acute or Chronic will feed straight into the summation method. Details of the summation method are described in 2.9.3.4.6.1 to 2.9.3.4.6.4.1.
- 2.9.3.4.5.2
-
Mixtures may be made of a combination of both ingredients that are classified (as Acute 1 and/or Chronic 1, 2) and those for which adequate toxicity test data are available. When adequate toxicity data are available for more than one ingredient in the mixture, the combined toxicity of those ingredients shall be calculated using the following additivity formulas (a) or (b), depending on the nature of the toxicity data:
Based on acute aquatic toxicity:
\(\frac{\sum{C_i}}{L(E)C_{50m}} = \sum\limits_n\frac{C_i}{L(E)C_{50i}}\)
where:
- Ci
- = concentration of ingredient i (mass percentage);
- L(E)C50i
- = LC50 or EC50 for ingredient i (mg/L);
- n
- = number of ingredients, and i is running from 1 to n; and
- L(E)C50m
- = L(E)C50 of the part of the mixture with test data
The calculated toxicity shall be used to assign that portion of the mixture an acute hazard category which is then subsequently used in applying the summation method;
Based on chronic aquatic toxicity:
\(\frac{\sum{C_i} + \sum{C_j}}{EqNOEC_m} = \sum\limits_n\frac{C_i}{NOEC_i} + \sum\limits_n\frac{C_j}{0.1 \times NOEC_j}\)
where:
- Ci
- = concentration of ingredient i (mass percentage) covering the rapidly degradable ingredients;
- Cj
- = concentration of ingredient j (mass percentage) covering the non-rapidly degradable ingredients;
- NOECi
- = NOEC (or other recognized measures for chronic toxicity) for ingredient i covering the rapidly degradable ingredients, in mg/L;
- NOECj
- = NOEC (or other recognized measures for chronic toxicity) for ingredient j covering the non-rapidly degradable ingredients, in mg/L;
- n
- = number of ingredients, and i and j are running from 1 to n;
- EqNOECm
- = equivalent NOEC of the part of the mixture with test data;
The equivalent toxicity thus reflects the fact that non-rapidly degrading substances are classified one hazard category level more "severe" than rapidly degrading substances.
The calculated equivalent toxicity shall be used to assign that portion of the mixture a long-term hazard category, in accordance with the criteria for rapidly degradable substances (table 2.9.1 (b)(ii)), which is then subsequently used in applying the summation method.
- 2.9.3.4.5.3
- When applying the additivity formula for part of the mixture, it is preferable to calculate the toxicity of this part of the mixture using for each ingredient toxicity values that relate to the same taxonomic group (i.e. fish, crustacea or algae) and then to use the highest toxicity (lowest value) obtained (i.e. use the most sensitive of the three groups). However, when toxicity data for each ingredient are not available in the same taxonomic group, the toxicity value of each ingredient shall be selected in the same manner that toxicity values are selected for the classification of substances, i.e. the higher toxicity (from the most sensitive test organism) is used. The calculated acute and chronic toxicity shall then be used to classify this part of the mixture as Acute 1 and/or Chronic 1 or 2 using the same criteria described for substances.
- 2.9.3.4.5.4
- If a mixture is classified in more than one way, the method yielding the more conservative result shall be used.
- 2.9.3.4.6
- Summation method
- 2.9.3.4.6.1
- Classification procedure
- 2.9.3.4.6.1.1
- In general a more severe classification for mixtures overrides a less severe classification, e.g., a classification with Chronic 1 overrides a classification with Chronic 2. As a consequence the classification procedure is already completed if the results of the classification is Chronic 1. A more severe classification than Chronic 1 is not possible; therefore, it is not necessary to pursue the classification procedure further.
- 2.9.3.4.6.2
- Classification for category Acute 1
- 2.9.3.4.6.2.1
- First, all ingredients classified as Acute 1 are considered. If the sum of the concentrations (in %) of these ingredients is greater than or equal to 25% the whole mixture shall be classified as Acute 1. If the result of the calculation is a classification of the mixture as Acute 1, the classification process is completed.
- 2.9.3.4.6.2.2
-
The classification of mixtures for acute hazards based on this summation of the concentrations of classified ingredients, is summarized in table 2.9.3 below.
Table 2.9.3 - Classification of a mixture for acute hazards based on summation of the concentrations of classified ingredients Sum of the concentrations (in %) of ingredients classified as: Mixture is classified as: Acute 1 x M* ≥25% Acute 1 * For explanation of the M factor, see 2.9.3.4.6.4.
- 2.9.3.4.6.3
- Classification for categories Chronic 1 and 2
- 2.9.3.4.6.3.1
- First, all ingredients classified as Chronic 1 are considered. If the sum of the concentrations (in %) of these ingredients is greater than or equal to 25% the mixture shall be classified as Chronic 1. If the result of the calculation is a classification of the mixture as Chronic 1 the classification procedure is completed.
- 2.9.3.4.6.3.2
- In cases where the mixture is not classified as Chronic 1, classification of the mixture as Chronic 2 is considered. A mixture shall be classified as Chronic 2 if 10 times the sum of the concentrations (in %) of all ingredients classified as Chronic 1 plus the sum of the concentrations (in %) of all ingredients classified as Chronic 2 is greater than or equal to 25%. If the result of the calculation is classification of the mixture as Chronic 2, the classification process is completed.
- 2.9.3.4.6.3.3
-
The classification of mixtures for long-term hazards based on this summation of the concentrations of classified ingredients is summarized in table 2.9.4 below.
Table 2.9.4 - Classification of a mixture for long-term hazards based on summation of the concentrations of classified ingredients Sum of the concentrations (in %) of ingredients classified as: Mixture classified as: Chronic 1 x M* ≥25% Chronic 1 (M x 10 x Chronic 1) + Chronic 2 ≥25% Chronic 2 * For explanation of the M factor, see 2.9.3.4.6.4.
- 2.9.3.4.6.4
- Mixtures with highly toxic ingredients
- 2.9.3.4.6.4.1
-
Acute 1 or Chronic 1 ingredients with acute toxicities well below 1 mg/L and/or chronic toxicities well below 0.1 mg/L (if non-rapidly degradable) and 0.01 mg/L (if rapidly degradable) may influence the toxicity of the mixture and are given increased weight in applying the summation method. When a mixture contains ingredients classified as Acute 1 or Chronic 1, the tiered approach described in 2.9.3.4.6.2 and 2.9.3.4.6.3 shall be applied using a weighted sum by multiplying the concentrations of Acute 1 and Chronic 1 ingredients by a factor, instead of merely adding up the percentages. This means that the concentration of "Acute 1" in the left column of table 2.9.3 and the concentration of "Chronic 1" in the left column of table 2.9.4 are multiplied by the appropriate multiplying factor. The multiplying factors to be applied to these ingredients are defined using the toxicity value, as summarized in table 2.9.5 below. Therefore, in order to classify a mixture containing Acute 1 and/or Chronic 1 ingredients, the classifier needs to be informed of the value of the M factor in order to apply the summation method. Alternatively, the additivity formula (2.9.3.4.5.2) may be used when toxicity data are available for all highly toxic ingredients in the mixture and there is convincing evidence that all other ingredients, including those for which specific acute and/or chronic toxicity data are not available, are of low or no toxicity and do not significantly contribute to the environmental hazard of the mixture.
Table 2.9.5 - Multiplying factors for highly toxic ingredients of mixtures Acute toxicity M factor Chronic toxicity M factor L(E)C50 value NOEC value NRD* ingredients RD† ingredients 0.1 < L(E)C50 ≤ 1 1 0.01 < NOEC ≤ 0.1 1 - 0.01 < L(E)C50 ≤ 0.1 10 0.001 < NOEC ≤ 0.01 10 1 0.001 < L(E)C50 ≤ 0.01 100 0.0001 < NOEC ≤ 0.001 100 10 0.0001 < L(E)C50 ≤ 0.001 1000 0.00001 < NOEC ≤ 0.0001 1000 100 0.00001 < L(E)C50 ≤ 0.0001 10000 0.000001 < NOEC ≤ 0.00001 10000 1000 (continue in factor 10 intervals) (continue in factor 10 intervals) * Non-rapidly degradable.
† Rapidly degradable.
- 2.9.3.4.6.5
- Classification of mixtures with ingredients without any useable information
- 2.9.3.4.6.5.1
- In the event that no useable information on acute and/or chronic aquatic toxicity is available for one or more relevant ingredients, it is concluded that the mixture cannot be attributed (a) definitive hazard category(ies). In this situation the mixture shall be classified based on the known ingredients only.
- 2.9.4
-
Lithium batteries
Cells and batteries, cells and batteries contained in equipment, or cells and batteries packed with equipment, containing lithium in any form shall be assigned to UN Nos. 3090, 3091, 3480 or 3481 as appropriate. They may be transported under these entries if they meet the following provisions:
Each cell or battery is of the type proved to meet the requirements of each test of the Manual of Tests and Criteria, part III, sub section 38.3. Cells and batteries manufactured according to a type meeting the requirements of sub-section 38.3 of the Manual of Tests and Criteria, revision 3, amendment 1 or any subsequent revision and amendment applicable at the date of the type testing may continue to be transported, unless otherwise provided in this Code.
Cell and battery types only meeting the requirements of the Manual of Tests and Criteria, revision 3, are no longer valid. However, cells and batteries manufactured in conformity with such types before 1 July 2003 may continue to be transported if all other applicable requirements are fulfilled.
Note: Batteries shall be of a type proved to meet the testing requirements of the Manual of Tests and Criteria, part III, subsection 38.3, irrespective of whether the cells of which they are composed are of a tested type.
Each cell and battery incorporates a safety venting device or is designed to preclude a violent rupture under conditions normally incident to transport.
Each cell and battery is equipped with an effective means of preventing external short circuits.
Each battery containing cells or series of cells connected in parallel is equipped with effective means as necessary to prevent dangerous reverse current flow (e.g., diodes, fuses, etc.).
Cells and batteries shall be manufactured under a quality management programme that includes:
a description of the organizational structure and responsibilities of personnel with regard to design and product quality;
the relevant inspection and test, quality control, quality assurance, and process operation instructions that will be used;
process controls that should include relevant activities to prevent and detect internal short circuit failure during manufacture of cells;
quality records, such as inspection reports, test data, calibration data and certificates. Test data shall be kept and made available to the competent authority upon request;
management reviews to ensure the effective operation of the quality management programme;
a process for control of documents and their revision;
a means for control of cells or batteries that are not conforming to the type tested as mentioned in (.1) above;
training programmes and qualification procedures for relevant personnel; and
procedures to ensure that there is no damage to the final product.
Note: In house quality management programmes may be accepted. Third party certification is not required, the procedures listed in .1 to .9 above shall be properly recorded and traceable. A copy of the quality management programme shall be made available to the competent authority upon request.
Lithium batteries, containing both primary lithium metal cells and rechargeable lithium ion cells, that are not designed to be externally charged (see special provision 387 of chapter 3.3) shall meet the following conditions:
the rechargeable lithium ion cells can only be charged from the primary lithium metal cells;
overcharge of the rechargeable lithium ion cells is precluded by design;
the battery has been tested as a lithium primary battery; and
component cells of the battery shall be of a type proved to meet the respective testing requirements of the Manual of Tests and Criteria, part III, subsection 38.3.
- "Except for button cells installed in equipment (including circuit boards), manufacturers and subsequent distributors of cells or batteries manufactured after 30 June 2003 shall make available the test summary as specified in the Manual of Tests and Criteria, Part III, subsection 38.3, paragraph 38.3.5.